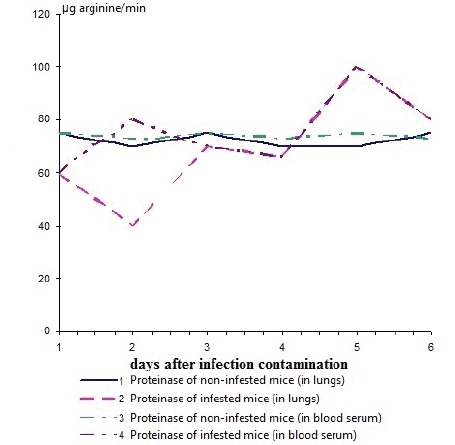
Figure 1: Dynamics of change proteinase activity in lungs and blood serum in white mice infested with a flu virus А/PR/8/34 in flow of 6 days (the total data of experiences with mice in mass of 6-9 g and 16-17 is presented)

Divocha VA1* Basarab YA2
1Ukrainian scientific research institute of medicine of transport, Odessa, Ukraine*Corresponding author: Divocha VA, Ukrainian scientific research institute of medicine of transport, Odessa, Ukraine, E-mail: divocha09@ukr.net
The present study examines the impact of an experimental influenza infection when outbred white mice were infested by a flu virus on the changes of proteinase activity in pulmonary tissue and blood serum of animals. These materials study concurrently the activity of this proteinase inhibitor.
Flu virus; Trypsin-like proteinase; Inhibitor of proteinase
Interaction of a virus and a cell is a single whole, representing a set of intrinsic relations of two opposite principles. The net result of this interaction is genetically defined both by the owner and the originator and depends on regulation of both the process of virus reproduction and protective forces of an organism. The balance between these processes determines the interaction outcome: the destruction of the owner, its absolute recovery or the formation of the chronic form of an infection contamination [1-6].
It is known that in replication of a flu virus, the precursor of the hemagglutinin is synthesised, which splitting on big and small subunit is necessary for acquisition of infectious activity by a virus. The splitting is carried out by cellular trypsin-like proteinases [7-11]. During influenza infection there is a change of specific cellular proteinases evidencing cooperative interaction of virus proteins with cellular components. Most likely, the dynamics of proteinase level change is bound to a reproduction of the virus and its proteolytic activation by the same proteinases. However, this phenomenon is too little studied; there are only the reports [12] about down regulation of proteinase activity in early periods and its increase in later periods of infection upon infestation of chicken embryos with viruses of flu and Sendai [12].
The purpose of this work was to study the action of experimental influenza infection in white mice and chicken embryos infested with flu viruses A on the dynamics of proteinase and inhibiting activity.
In researches, flu virus А/РR/8/34 (Н1N1), adapted for a pulmonary tissue of white mice (the virus was received from laboratory of museum strains of Institute of virology of D.I.Ivanovskiy of the Russian Academy of Medical Science) was used. The infectious titre of virus was 7 lg EID50/0.2 ml. Besides, we used white outbred mice weighing 7-9 and 16-17g. The animals were infested by А/РR/8/34 in intranasal route in volume of 0.05 ml under an easy ether narcosis diluted 10-2 that corresponded to 20 LD50 infectious virus dose. Such dose provided 100% destruction of animals in 6 days after infestation. The animals (5 mice in each group) were slaughtered, their lungs and blood were removed 15, 30 minutes, 1.6 h. and further in 1-6 days after infestation. The lungs were flushed twice in cold phosphate buffer рН 7.5 and triturated in a mortar, suspended in the buffer (1 ml per 1 lung), homogenised by ultrasound in mode 7 of teh device High Intensity Ultrasonic Procession, Chicago, «Cole Parmel» (USA), centrifuged at 10000 rpm on whizzer RC5c, manufactured by «Sorvall Instruments», rotor SS-34, 1 hour, at +4°С. Supernatant and blood serum was used for definition of activity: infectious, proteinase and inhibiting proteinase. Activity of a trypsin-like proteinase was determined by K.M.Veremeenko method [13] modified by S.V.Vovchuk [14]. Identification of activity of proteinase inhibitors in homogenates of lungs and in blood serum made the caseic method previously described by A.P.Levitsky [15]. Infectious titre of virus in lungs of the infected mice was determined by infestation of 9-10 days chicken embryos and expressed in lg EID50/0.2 ml. Hemagglutination reaction was made under the standard method.
The received data were processed using Microsoft®Excel software.
In lungs and blood serum of uninfected animals, the level of a trypsinlike proteinase and its inhibitor was not exposed to evaluated changes during the observation period (6 days) and was in balance (Figure 1).

Figure 1: Dynamics of change proteinase activity in lungs and blood serum in white mice infested with a flu virus А/PR/8/34 in flow of 6 days (the total data of experiences with mice in mass of 6-9 g and 16-17 is presented)
During intranasal infestation of mice by flu virus of А/PR/8/34 (Н1N1) strain, it was revealed that starting from 15 minutes and up to 6 h after infestation, the proteinase activity in lungs and blood serum decreased, and activity of an inhibitor, on the contrary, raised, especially in lungs (Figure 1). Hemagglutination and infectious activity during the first 6 h after infestation was not determined.
During the subsequent period, the proteinase activity increased, and by 3rd day reached a reference level. During this period, hemagglutination and infectious activity sharply increased. By 48 h after infestation, infectious and hemagglutination activity reached its maximum value (Figure 2). By 96 h after infestation, infectious and hemagglutination activity sharply decreased and the mortality of infested animals began. The infested animals who remained alive by the 4th day after infestation, the repeated increase of proteinase activity by 5-6th day became perceptible, thus both infectious and hemagglutination activity was repeatedly increased. By 6th day, control group of flu virus animals perished.
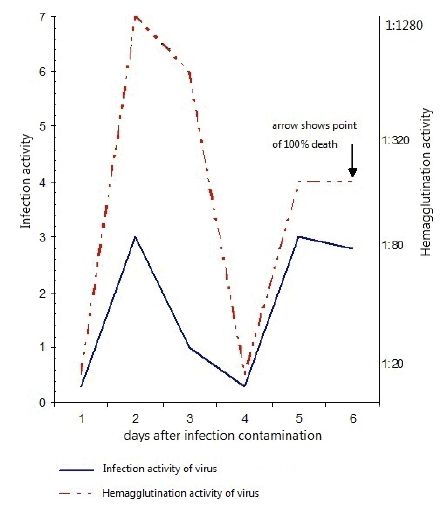
Figure 2: Infection and hemagglutination activity of a homogenate in the lungs of white mice infested with a flu virus А/PR/8/34 in allantoic fluid of chicken embryos
Thus, the lungs of the infested mice had a correlation between accumulation of infectious virus and depression of proteinase activity by the 2nd day after infestation. At the same time, the augmentation of virus titer by the 5th day in a pulmonary tissue and blood serum of infested animals also coincided with rising proteinase activity.
In search of the causes of proteinase activity depression on the 1st day after infestation, we determined inhibiting proteinase activity both in pulmonary tissue and blood serum of white mice.
As we can see on figure 3, in not infested animals, proteinase activity and proteinase inhibiting activity in blood serum were at stable level. In a pulmonary tissue, proteinase inhibiting activity was found in very insignificant quantities.
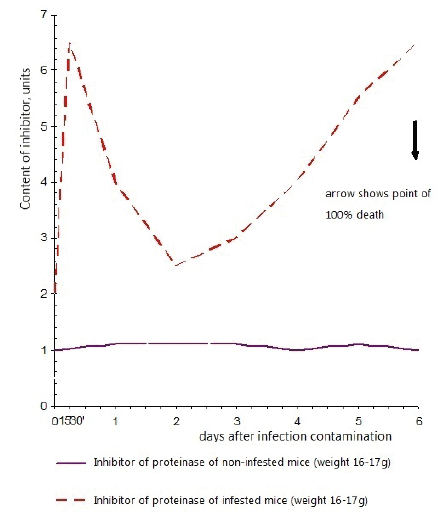
Figure 3: Activity of an inhibitor of trypsin-like proteinases in lungs of mice infested with flu virus А/PR/8/34
In pulmonary tissue and blood serum of infested animals, proteinase inhibiting activity after infestation sharply increased by 6 h and dropped by 2nd day, and then gradually rose by 5th day.
Thus, it is possible to explain the depression of proteinase activity during the first hours after infestation by augmentation of proteinase inhibiting activity (it was clearly observed in pulmonary tissue of infested mice), and it’s rising by 2-3rd days of development of infection contamination by depression of inhibiting activity. However, simultaneous increase of proteinase and inhibiting activity by the 5th day after infestation evidence other causes of proteinase activation in this period development of infection.
The cited data shows that in blood serum of not infested animals, the balance of proteinase activity and proteinase inhibiting activity was broken at infestation by a flu virus. It is possible to secure three periods in infectious process which are characterised by different degree of virus reproduction, level of proteinase activity and level of proteinase inhibiting activity.
The most critical changes occurred during the first hours after infestation. 6 h after infestation, the quantity of proteinase both in lungs and in serum of the infested animals dropped, and proteinase inhibiting activity increased. Thus, it is possible to assume that reduction of proteolytic activity is related to the respective accumulation of proteinase inhibitor. Apparently, flu virus infested cells induce appearance of inhibitor both in pulmonary tissue, and in blood serum.
During the period of the biggest accumulation of infectious virus (2 days after infestation), proteolytic activity dropped, however, this decrease was accompanied by reduction of inhibitor activity. Probably, one of the causes is use of cellular trypsin-like proteinases for proteolytic virus activation in the lungs of the infested mice and pool deep in infested animal’s organism. Repeated decrease of proteinase activity by 5-6 days is caused by other factors, apparently, by bacterial flora overlay.
Download Provisional PDF Here
Article Type: Research Article
Citation: Divocha VA (2016) Dynamics of Inhibitors and Proteinases at the First Stage of the Mice Organism Lesion with Experimental Influenza Infection Contamination. Autoimmun Infec Dis 2(3): doi http://dx.doi.org/10.16966/2470-1025.118
Copyright: © 2016 Divocha VA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access