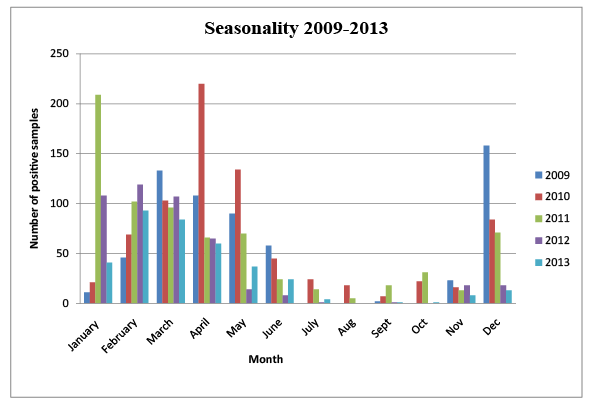
Figure 1: Analysis of the Genotype of the selected isolates

Said Al Baqlani*1 Salah AI Awaidy2 Zainab AI Lawati1 Mohammed Al Toubi1 Fatima Sajina1 Nadia Teleb3 Suleiman AI Busaidy1
1Central Public Health Laboratories, DGHA-MOH, Muscat, Oman*Corresponding author: Said Al Baqlani, Central Public Health Laboratories, DGHA-MOH, Muscat, Oman, Tel: +96899248132; E-mail: saidalisaif@gmail.com
Oman is on the verge of introducing rotavirus vaccination for children. It will be part of the National EPI programme based on the data collected on the burden of disease and circulating rotavirus genotypes. In 2005 the predominant circulating genotype in Oman was G1P[8] but strain prevalence changes over time have been observed. (Observation briefly mentioned in the discussion)
To receive more detailed information a 5 year study from January 2009 to December 2013 has been conducted to elucidate the temporal diversity of genotypes and to provide more recent information on the burden of rotavirus infection among hospitalized children less than five years of age.
A total of 6034 stool samples from hospitalized Oman and non Oman children less than 5 years of age with moderate to severe diarrhoea were collected in 12 regional hospitals, geographically representing the whole of Oman. In this study 48.5 % (2931/6031) of the children with clinical signs of diarrhoea suffered from rotavirus associated diarrhoea. Molecular characterization was done on 450 rotavirus antigen positive samples selected on basis of local regional distribution.
The main genotype combinations were G1P[8], G2P[4], and G9P[8]. G1P[8] was the dominant rotavirus in 2009 21/77 (27%), in 2011 32/66 (48.5%) and in 2012 36/65 (55.38%), whereas in 2010 and 2013 the predominant genotype was G2P[4] 13/77 (16.9%) and 23/50 (46%) respectively. Several strains exhibited unusual combinations of G and P genotypes indicating the likelihood of natural re-assortment. The unusual P[10] genotype seen in our previous study (Said et al; 2005) was not detected in the current study, however, there was an increase of G9 sequences since 2007. It is noteworthy to mention the detection of G12 for the first time in Oman.
In light of the observation that the disease and economic burden on rotavirus induced diarrhoea decreased in countries which implemented rotavirus vaccination programmes, the high prevalence of rotavirus associated diarrhoea in Oman implies the introduction of rotavirus vaccination.
Rotavirus burden; Rotavirus genotypes
Diarrhoea is a significant cause of morbidity and mortality worldwide. Especially affected are infants and young children below five years of age. In a variety of investigations, attempts were made to estimate the burden of bacterial, parasitic and viral agents responsible for episodes of diarrhoea in a group of children [1]. It was determined that globally, rotavirus remains a major cause of morbidity and mortality in developing countries. Dependent on the studies included in the extrapolation of the worldwide burden of rotavirus infections of children up to approximately 500 000 annual deaths due to rotaviruses were calculated [1,2] .
In 2004 World Health Organization-Eastern Mediterranea Region Office (WHO-EMRO) recommended to member countries to establish National Rotavirus Surveillance Programme with the objective of documenting burden of rotavirus gastroentiritis genotyped and using the data for evidence-based decision making on the introduction of rotavirus vaccine.
In 2004 WHO-EMRO established the Eastern Mediterranean Rotavirus Surveillance Network in the same year. In the following year (2005) Oman was one of the first EMR countries to establish a national surveillance system to screen for rotavirus infections in order to determine the disease burden and circulating genotypes.
Human rotavirus as cause of gastrointestinal infections and diarrhoea were first described in 1973. The importance of the pathogen has initiated extensive research on the structure, and the replication of the virus as well as of the pathogenesis and the immune responses [3,4]. Rotavirus infections range from subclinical infection to severe gastroenteritis with diarrhoea, vomiting, and lethal dehydration. The major protective immune response against rotavirus is directed against the two outer capsid proteins VP7, a glycoprotein (G protein), and VP4, a protease-sensitive protein (P protein). Both proteins are used to type strains, which have been shown to vary over time within geographic locations and from region-to-region .Molecular epidemiological studies distinguish currently 27 G-genotypes and 35 P-genotypes. Of major worldwide epidemiological importance are the genotypes G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and to a lesser extent G12P[8] [5].
After the development and introduction of rotavirus immunization in many countries it was shown that the number of children suffering from rotavirus infections can be significantly reduced as shown by a decline in hospital admissions and deaths of children below the age of 5 years [6,7].
Rotavirus infections are transmitted through the faecal-oral route and are seen throughout the year in Oman. Although the incidence of infection in children in industrialized and developing countries is similar, outcomes vary widely but the case fatality rate is higher in the poor developing countries in Africa and Asia.The risk of dying from rotavirus disease before age 5 years is 1 in 50,000 in industrialized countries, but in developing countries it can be as high as 1 in 200 [8]. The mortality rate due to rotavirus infection in Oman is unknown; however, the exposure of children under five years of age could be same as seen in other studies which have determined that almost every child becomes infected by rotavirus before reaching five years of age [9].
Rotavirus viral antigens VP7 and VP4 are considered as important targets for vaccine development, because they are immunogenic and elicit protective neutralizing antibodies [10]. Two live attenuated rotavirus vaccines have been developed and licensed [11]. Vaccination was introduced in various countries, including the United States and Latin America [12], Europe [13] and the Middle East (path.org/rotavirusvaccines 2014). Vaccination against rotavirus infections has demonstrated a great health benefit by reducing diarrhoea episodes in both developed and developing countries [14]. The two vaccines have been developed on the basis of different rationales for the mechanism of protection. The Rotarix vaccine (GSK Biological) has been developed on the basis of the expectation that repeated immunizations with the monovalent vaccine will elicit a broad, heterotypic cross-protection [15,16]. On the other hand, the RotaTeq vaccine (Merck) has been developed to elicit an immune response that has as broad a range of neutralizing antibodies as possible, including the major neutralizing antibodies in a pentavalent reassortant vaccine [17-19].
WHO established in 2008 The Global Rotavirus Surveillance Network (GRSN) with the aim to generate local data for decision making regarding rotavirus vaccine introduction and sustained use, to assess and monitor disease trends and genotype distribution over time and to develop a platform for vaccine effectiveness studies (WHO 2009).Despite the global awareness of rotavirus infection and disease, little has been published on the impact of rotavirus on the health of Omani children. Scrimgeour et al. [20] reported on the epidemiological meta-analysis of infectious and tropical diseases in the Sultanate of Oman. These authors found a nearly 50% reduction in the incidence of gastroenteritis and diarrhoea in Oman between 1992 and 1998 but did not report on the viral etiological agents involved with diarrhoea in that study. Later Al Baqlani et al. [21]reported that approximately 57% of all children under 5 years hospitalised with diarrhoea were infected with rotavirus. The main genotypes circulating in Oman during the study period (2005) was GIP[8], followed by G2P[4], and G3P[8]. Worldwide significant changes of genotypes circulating in different regions at a given time were observed and the appearance of new genotypes has been described [22]. The molecular epidemiological studies performed in Oman are important for survey of the epidemiological trend over time and serve as basis for the evaluation of the impact and efficiency of rotavirus vaccination of children in Oman.
The sentinel surveillance for diarrhoea in children 1-59 months of age was established using the procedures outlined in the generic WHO protocol on rotavirus surveillance. On the basis of the population census and regional representation, all twelve regional hospitals in the country were selected as rotavirus surveillance sites geographically representing the whole of Oman.
Between January 2009 and December 2013 all children <5 years old who were hospitalized in 12 regional hospitals for acute watery diarrhoea (defined as ≥ 3 liquid or semi-liquid stools in 24 hours) were enrolled in the surveillance program. A standard data entry form was completed upon enrolment.
Six thousand and thirty four (6034) stool specimens (5 ml or 1 gm) were collected in screw top containers within 48 hours of admission to exclude children with nosocomial infection. All stool specimens were initially stored at 2°to 8o C until transportation observing cold chain to the Central Public Health Laboratory (CPHL), Department of Laboratories, Ministry of Health, Muscat, Oman. Samples were tested by antigen-based ELISA and stool aliquots stored at -20°C until further analysis. Rectal swabs in bacterial culture media were not included in the study.
Ten-percent (10%) suspensions of each stool specimen were prepared in phosphate-buffered solution (PBS) containing antimicrobial agent and detergent and screened using enzyme-linked immunosorbent assay (ELISA) kit from OXOID (Ely), ProSpecT Rotavirus Microplate Assay for the presence of rotavirus antigen.
Four hundred and fifty (450) rotavirus antigen ELISA-positive samples were selected for genotyping. The selection was based on the location of the health institution (geographical area), the severity of the disease, demographic information and season. Severity categorized by the paediatrician following the guidelines in the Oman Integrated Management of Childhood Health 3rd Ed. Vol 1.6 page 22.
Group A Rotavirus dsRNA was extracted from the stool specimens using TRIzol-chloroform method as per the extraction SOP. Briefly, a portion of the 10% of stool suspension was thoroughly mixed by vortex and left to settle at Room Temperature (RT) for at least 30 minutes. Then 250 ul of clear supernatant was recovered and mixed with 750 ul of TRIzol reagent in a 1.5 ml Eppendorf tube. It was vortexed thoroughly and incubated for 5 minutes at ambient temperature, followed by the addition of 200 ul chloroform, vortexed again to mix and incubated at ambient temperature for 3 minute. After centrifugation at 12,000 rpm for 5 minutes, 450 ul of supernatant was transferred to a new Eppendorf tube and 700 ul of icecold isopropyl alcohol was added. After gentle mixing and incubation at ambient temperature for 20 minutes the sample was again centrifuged at 12,000 rpm at 4°C for 15 minutes to pellet the dsRNA. The supernatant was discarded and the pellet was air-dried at room temperature. The pellet was suspended in 40 ul sterile de-ionized or RNase free sterile water and stored at -20°C until needed for the RT-PCR assays.
The genotypes of the rotavirus strains were determined by RT-PCR using multiplex primers for G and P typing as previously described[23,24. Briefly, RNA was amplified by a two-step standard RT-PCR using specific primers and methods as described elsewhere [23,24]. A few numbers of specimens which showed visible bands in agarose gel electrophoresis in the first RT-PCR were untypable in the following reaction.
The untypable specimens were subjected to sequencing utilizing sequencing primer. The cDNA products were cleaned, (using Promega kit, Wizard SV gel clean-up kit) cycle sequenced and subjected to sequencing using Sanger’s method [25]. (Sequences were analysed by DNAStar-Laser gene (SeqMan) software and a nucleotide BLAST search was done to determine the rotavirus genotypes.
Figure 1 shows the distribution of rotavirus diarrhoea cases during the study period. The scenario is comparable to the seasonal distribution of rotavirus infection in other countries with the peak of infections in the cooler months and low number of cases in hot months.
From 6034 stool specimens investigated 2931 (48.5%) were rotavirus antigen positive by ELISA. A total of 450 of the 2931 positive samples (15.3%) representative for all the geographical regions of Oman and of good integrity were selected for further analysis to determine their genotypes. In 240 samples the VP7 and VP4 genotype could be determined as shown in Table 1. Untypable samples to determine VP7 and VP4 genotypes are not included in this Table.
VP7 genotyping: First round RT-PCR amplification products (1062 bp of the VP7 gene) were obtained in 266/450 (59%) of the analyzed samples utilizing primer sets [23]. The genotype of VP7 (G) could be determined in 224/266 (84.2%) samples by PCR and agarose gel electrophoresis. The remaining 42/266 (15.7%) untypable samples were subjected to Sanger’s sequencing methodology. Genotypes from 16 of 42 samples were determined by sequencing using sBeg9 and End9 primers [23]. These were four G1, three G2, two G9, one G3, one G4, two G1/G4 and three G12. The remaining 26 of 42 were untypable by our existing methodologies. The most frequently VP7 genotype detected was G1 followed by G2, G9 and G3 and few strains gave G4 and G12 genotypes.
The predominant genotype detected in the years 2009, 2011 and 2012 was G1 and in 2010 and 2013 the predominant was G2. We observed an increase of rotavirus genotype G9 in 2011 whereas the genotypes G3, G4 and G12 circulated at a comparable low level in the study period. Mixed infections between G1, G2 and G3 were seen in 20/240 (8.3%) of the genotyped samples.
First round RT-PCR amplification product (876 bp) of the VP4 gene was obtained in 275/450 (61.1%) of the samples using in use primer sets [24]. Out of the 275 RT-PCR first round positive samples, the VP4 (P) genotype of 219 samples (79.6%) were successfully determined using the standard conventional RT-PCR methods. The remaining 56 of 275 (20.4%) untypable samples were sequenced using first round RT-PCR (Con2 and Con3) primers [24] and 23 genotypes were determined i.e. three P[4], one P[6] and nineteen P[8].The remaining 33 samples could not be typed utilizing our locally existing methodologies.
The P genotype most frequently detected was P[8] followed by P[4] and only one isolate with the P[6] genotype was found. The dominance of genotype P[8] was throughout the period of study whilst strains with the P[4] genotype dominated in 2010, P[6] was detected only in 2009 in combination with the rare G12.
Table 1 shows the age distribution of rotavirus genotypes circulating in Oman. We observed the presence of commonly circulating rotavirus genotypes in all age groups except in children below 2 years of age as non of the rotavirus antigen-based ELISA positive in this age group were positive in the 1st RT-PCR. We also determined that G1 P[8] and G2 P[4] were circulating in all age groups except those under 2 months predominantly in 6-24 months age group. The distribution of genotypes by age group is in concordance with the ELISA results (Table 3).
Combinations of G and P genotypes: Molecular characterization of G and P was carried out on 240 rotavirus strains. Predominant G and P type combinations determined were G1P[8] 100 strains (41.7%), G2P[4] 52 strains (21.7%) G3 P[8] 16 strains (6.7&) and G9 P[8] 32 strains (13.3%) these four genotype combination accounted for more than 80% of all strains genotyped. for Mixed infections were observed in 20 samples (8.3%) mostly in the following combinations: G1G9,G2G3, G2G4, G2G9, G1G12 and G3G4 with no mixed infections in P genotypes. The remaining rare combinations accounted for less than 10 % as shown in (Table 2).

Figure 1: Analysis of the Genotype of the selected isolates
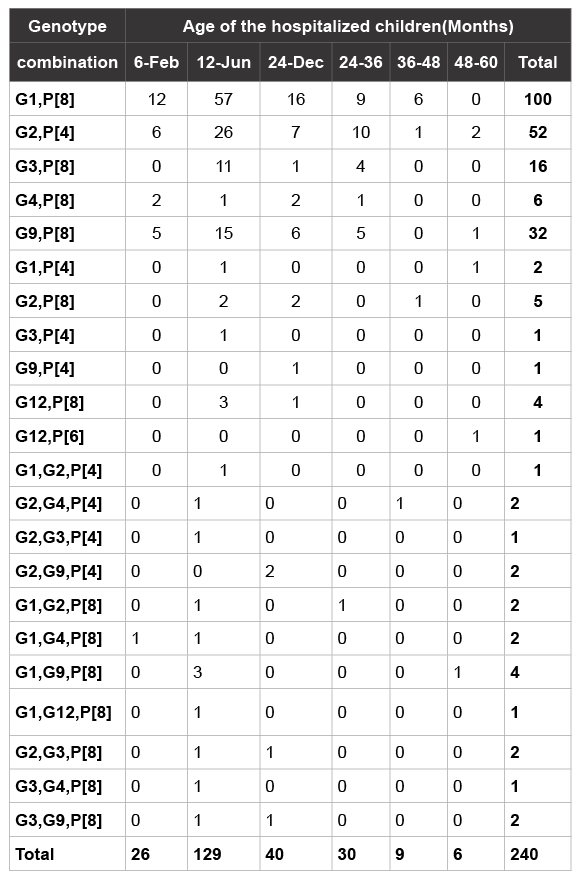
Table 1: Genotypes distribution by age groups
The purpose of this study was to estimate the rotavirus disease burden and determine the currently circulating genotypes between January 2009 and December 2013 by conducting a hospital-based surveillance of rotavirus gastroenteritis in hospitalized children under 5 years of age. This study was in line with the recommendations of WHO (The Global Rotavirus Surveillance Network) to generate country specific data for decision making regarding rotavirus vaccine introduction and to monitor disease trends and rota virus genotype distribution over time.
In a small preliminary study in Oman conducted from November 1990 to October 1992, 31 % of children <24 months of age with gastroenteritis were infected with rotavirus, compared with 6% of non-diarrheal controls [26]. However, no information on the genotypes circulating at that time in Oman are available. In a follow up investigation conducted in 2005 it was shown that approximately 57% of children younger than 5 years suffering from gastrointestinal diseases tested positive for rotavirus antigen [21] where genotyping revealed that genotype combinations G1P[8], G3P[8] and G2P[4] were the predominant circulating genotypes. In a follow-up study in the years 2006-2007 the majority of the isolates belonged to the genotype G2, followed by G1 and G9 [27]. The main P-genotype observed in this investigation were P[8] P[4] and P[10] respectively.
Between 2006 and 2008 the predominant genotypes were G2 and P[4], as it accounted for more than 45% (109/226) of all positive samples genotyped (data not shown).
In our current study 48.5% (2931/6031) children with diarrhoea tested positive for rotavirus antigen. Genotyping revealed comparable results with G1P[8] (2009, 2011 and 2012) and G2P[4] (2010 and 2013) being the predominant genotypes . The G genotype frequently seen in this study was G1 constituting 42.5% of all samples followed by G2 (23.8%), G9 (13.8%), G3 (7.1%), G4 (2.5%) and G12 (2.1%) the remaining percentage being G combinations. Most of the G genotypes were found in combination with P[8] or P[4] in the P genotypes pattern. This is being seen in other parts of the world [28]. Two important observations were documented in the G genotyping. Firstly, in comparison to the 2005 investigation there was a significant increase of G9 genotype that accounted for almost 14% of G genotype. This increase of G9 has been seen in other countries as well [29]. Secondly, for the first time we identified G12 in a few samples collected in 2009 and 2013. Other countries in the Middle East have identified G12 but also in low level of circulation [30]. Infection that involve mixtures of genotypes were seen in 20/240 (8.3%) samples. It was observed that more than 91% of rotavirus strains belonged in the 5 globally common G genotypes (G1, G2, G3, G4, and G9) and more than 99% of P genotypes were within P[4] and P[8]. Further epidemiological studies on archived samples have to be performed to get more information on the prevalence and epidemiology of genotype G12 using specific primers for the detection of G12 sequences.
In this 5 year study period we found that VP4 genotypes predominantly circulating in Oman are P[8] and P[4] and very rarely P[6]. The P genotypes pattern identified after the characterization of VP4 gene does not differ very much from what we had observed in 2005 or global observations 4. For P genotypes as seen in this study P[8] was identified as the predominant genotype, (73.7%,) followed by P[4], (25.8%)) and only one P[6] (0.4%) as shown in Table 1. Few samples could not be typed by the employed multiplex and sequencing methods. This has been reported elsewhere from studies in Africa and in Asia [31] and is probably due to inhibitory factors in stool, mismatched primer sets or emerging novel strains.
The most predominant G-P genotype combination observed was G1P[8],G9P[8] and G2P[4] which accounted for more than 76% of samples genotyped (Table 1).
Perhaps more significant was the number of rotavirus isolates with mixed genotype the highest seen in G1G9P[8] combination (4 out 20 samples). This fact is epidemiologically important, because mixed rotavirus infections are a prerequisite for reassortment in vivo or in vitro. Unusual G and P combination such as G1P[4], G2P[8], G3P[4] or G9P[4] were observed in a very low numbers 9/240 (3.75%) and mostly in combination with P[8]. As shown in Table 1, 169/240 of the rotavirus related diarrhoea cases were seen in children between 6 and 24 months of age. G1P[8] accounted for approximately half of the infections (73/169) in this age group. A high prevalence of G9P[8] was also observed in the same age group (21/169).
In principal this investigation has shown that there is no significant decrease in disease burden on rotavirus related diarrhoea episodes in Oman despite improved health facilities, sanitation and infection control measures. Vaccination against rotavirus not yet introduced in Oman so introduction of rotavirus vaccination in Oman children will definitely have an impact on the reduction of disease and economic burden [32].
This study has elucidated the magnitude of rotavirus related diarrhoea episodes in Oman. The total number of diarrhoea episodes due to various etiological agents in the under 5 years age group in Oman is approximately 75,000 episodes annually. It has been determined that Rotavirus accounts for more than 45% of all diarrhoea cases and predominantly in children between 12 and 24 months of age (Table 1). Because improved sanitation or better health services does not have a direct impact on the prevalence of rotavirus disease, and the rates of positive cases needing hospitalization remains high in Oman, the primary public health intervention as documented by other countries should be vaccination. In light of the observation that the burden and severity of rotavirus induced diarrhoea decreased in countries which implemented rotavirus vaccination programmes, the high prevalence of rotavirus associated diarrhoea in Oman implies the introduction of rotavirus vaccination of children in the country.
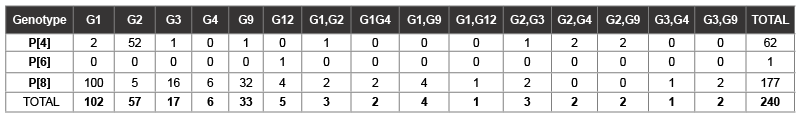
Table 2: Combination of G and P Genotypes
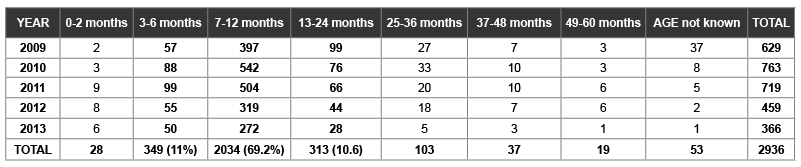
Table 3: Group A rotavirus antigen-based ELISA test positive by year and age group
The results of this study clearly justify the necessity for the introduction of rotavirus vaccine in Oman. An optimally effective rotavirus vaccine will avert a substantial proportion of childhood diarrhoea cases, severity/ mortality, and the associated health care resource use in Oman.
The two currently licensed rotavirus vaccines (RotaTeq and RotaRix) should have the same high degree of efficacy and safety as seen in other countries because of similarity of the Oman circulating genotypes and vaccine candidate genotypes.
However, constant evaluation of the vaccines is imminent because of the genomic diversity of rotaviruses and the existence of animal reservoir in human infections could undermine the vaccines efficacy. It has been observed that in low income countries the vaccines did not confer adequate efficacy and it was as low as 60% as shown in clinical trials in India, Malawi, Nicaragua Vietnam and Bangladesh and one of the reasons was strain diversity [33].
We appreciate great constructive comments from Prof. Dr. Georg Pauli and Prof. Dr. George Armah who reviewed our draft. Thanks to the MOH Health Institutions involved in the “National Rotavirus Surveillance Programme”.
None. This study was funded by Ministry of Health, Oman under the Central Public Health Laboratory budget.
Download Provisional PDF Here
Article Type: Research Article
Citation: Said Al Baqlani, Salah A Awaidy, Zainab AI Lawati, Mohammed Al Toubi, Fatima Sajina, et al. (2016) Molecular Characterization of Group A Rotavirus Genotypes Circulating in Oman between 2009 and 2013. J Emerg Dis Virol 2(2): doi http:// dx.doi.org/10.16966/2473-1846.113
Copyright: © 2016 Baqlani SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access