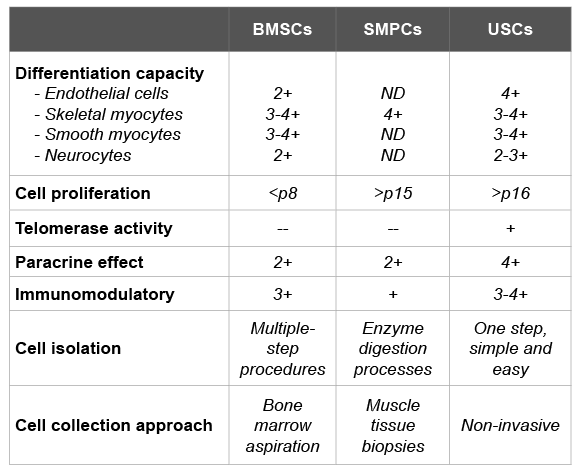
Table 1: Comparison of stem cell characters among USCs, SMSCs and BMSCs
Note: ND- not determined


Deying Zhang1,2 Jiaqiang Chu1 Wenyun Ma1 Mongjiao Gong1 Guanghui Wei1* Yuanyuan Zhang1,2*
1Department of Urology, Children Hospital of Changing Medial University, Changing, China*Corresponding author: Yuanyuan Zhang, Wake Forest University, Institute for Regeneration Medicine, Winston-Salem, NC, USA, Tel: 336-713-1189; E-mail: yzhang@wakehealth.edu Guanghui Wei, Department of Urology, Children’s Hospital of Chongqing Medial University, Chongqing, China
Stress urinary incontinence (SUI) is associated with the loss of various amounts of urine when intra-abdominal pressure increases due to muscle and nerve injury within the urethral sphincter. Injury due to vaginal delivery during childbirth is a primary cause of urinary incontinence in women, which results in damage to the external urethral sphincter, pelvic floor muscle, and the neural plexus posterolateral to the vagina [1,2]. SUI occurs in 19% of women younger than 45 years, 29% of older women, and in 78% of nursing home residents, and there are an increasing number of male patients as well [3,4]. SUI affects up to 13 million people in the United States and 200 million worldwide [5]. SUI decreases patients’ quality of life and is associated with significant morbidity. Besides pharmacotherapy [6], several invasive surgical therapies, including sling surgical procedures [7] and injection of bulking agents [8-15], have been commonly used to treat SUI. Although the sling procedure can reinforce weak pelvic floor muscles and has reported 71~73% success rates [7], the urethral sphincter deficiency remains [16]. Injection of bulking agents has provided encouraging outcomes, but over time these agents are absorbed and can cause chronic inflammation, per urethral abscesses, foreign body giant cell responses, erosion of the urinary bladder or the urethra, migration to inner organs, obstruction of the lower urinary tract with resultant urinary retention and severe voiding dysfunction, and even pulmonary embolism [15,17,18]. Cell-based therapy is an alternative to restore the urethral sphincter function in the treatment of SUI. Several autologous cell types, including mesenchymal stem cells (MSCs) derived from skeletal muscle [19-40], fat tissue [19,25,28,33,34,37,38,41-51], bone marrow [37,39,46,47,52-58] or umbilical cord blood mononuclear cells [59], have been used for this purpose. However, to obtain these cells, invasive procedures such as muscle tissue biopsy or bone marrow or fat aspiration are usually required, with an attendant risk of complications.
A subpopulation of cells isolated from urine possesses biological characteristics of stem cells [8,43-53], i.e. clonogenicity, high expansion capacity [60,61], multipotent differentiation capacity [8], proangiogenic paracrine effects [62], and immunomodulatory properties [63]. Thus, we have termed these cells “urine-derived progenitor/stem cells (USCs)” [64,45,50]. These cells are not MSC lineage but displayed surface markers that are similar to adult stem cells, i.e. positive staining for CD24, CD29, CD44, CD73, CD90, CD105, CD117, CD133, CD146, SSEA-4 and STRO- 1, and negative staining for CD14, CD31, CD34 and CD45 [61, 65]. USCs are easily induced pluripotent stem (iPS) cells [66], which provides a candidate strategy for personalized therapy. Multiple teams confirmed our results [67,68] and used human USCs for regeneration of various types of tissues and organs [69-79].
USCs possess multipotent differentiation capacity for tissue regeneration and give rise into potential to differentiate into desired cell lineages of all three germ layers [65], such as mesodermal (i.e. osteocytes, chondrocytes, adipocytes, myocytes and endothelial cells), and endodermal (i.e. podocyte, epithelial or urothelial cells), and ectodermal (i.e. neurocytes) cell types. For cell-based therapy for SUI, the implanted endothelial cells and myocytes are needed. USCs can differentiate give rise to functional endothelial cells with unique features: including in vitro vessel formation, endothelial cell marker expression, and barrier function with tight junction formation. In addition, USCs differentiate into skeletal muscle cells [65,80,81] and smooth muscle cell lineages [44,47,48]. These differentiated USCs displayed the morphology of myotubule-like or myofiber formation, in vitro contractility and expression of myogenic gene and protein markers in vitro and in vivo. More evidences have demonstrated that paracrine effect of stem cells play an important role in restore the urinary sphincter muscle function. USCs secrete higher levels of angiogenic growth factors and cytokines (i.e. IGF1, FGF1, PDGF, MMP9 and IL-8) than bone marrow MSCs. Our most recent studies further demonstrated the impact of the bioactive factors on recovery of sphincter function. In a rat model one week after vaginal distention injury, either local administration via periurethral injection of USCs or systemic administration via intraperitoneal injection significantly enhanced the sphincter function by increasing leak point pressure and restored the histology by protection against urethral sphincter injuries [82].
USCs most likely originate from kidney tissue [65]. These cells can be easily isolated and then generate a large number of cells from a single clone [60,61,65]. Additionally, up to 75% of the USCs collected from middle-aged individuals expressed telomerase activity (USCs-TA+) and retained long telomere length [83]. USCs-TA+ possessed higher proliferative capacities and were maintained for up to 67 population doublings, indicating that a single USC can generate up to 1.5 × 1020 (i.e. 267) cells within 14 weeks [61,65]. After optimizing our methods, 100-140 USC clones/24 hours’ urine was consistently obtained from each individual [60]. About 2-5 × 107 cells are needed for potential use in SUI therapy [58, 84-86]. Thus, one 200 ml urine sample can provide ample cells at the early stage (<p5) with a few weeks for the purposes of cell implantation.
As a new cell source, human USCs have the advantages [64,65] in cell differentiation and tropic factor section for urethra sphincter tissue regeneration, compared to skeletal muscle progenitor cells (SMPCs) or BMSCs (Table 1). In addition, USCs can generate more cells and survive longer (more than16 passages) as they express telomere activity but no teratomasneither tumors. Furthermore, USCs exist in the urine of each individual regardless of a person’s age, gender or race and offer enough amounts of cells for therapeutic injection. For cell isolation, purity of USCs can be guarantee as USC start from a single clone. Isolation processing is simple, which does not require by enzyme digestion. Furthermore, neither ethical issues nor immune reaction are involved in their use for tissue reconstruction when obtained from patients own urine sample, which makes easy get institutional ethical approval. It is also easy to isolate and culture USCs from other species, such as monkey, pig, dog and rabbits [87], which make these much feasible to use in animal experiments.

Table 1: Comparison of stem cell characters among USCs, SMSCs and BMSCs
Note: ND- not determined
To prevent SUI recurrence is critical after cell implantation. Although cell-based therapy with skeletal muscle progenitors has achieved promise outcomes for patients with SUI [58,88,89], multiple injections in some cases are needed [89]. Clearly, it is discouraged to take multiple muscle biopsies over times to continuously obtain skeletal muscle cells to treat the SUI recurrence. In contrast, USCs can be collected or repeatedly collected if needed with a simple, safe, low-cost and non-invasive procedure compared with invasive surgical biopsy procedures, which can avoid patient potential morbidity and complications, such as donor site trauma and infection. Therefore, it is no doubt those autologous USCs as an optimal cell source offer benefits over skeletal muscle progenitor or other MSCs to achieve a stable functional improvement over the longterm follow up in enhancement of sphincter tissue regeneration, or other tissue repair in urinary tract system.
The authors acknowledge funding support from National Natural Science Foundation of China (No. 81570650 and No. 81371704).
Download Provisional PDF Here
Aritcle Type: Short Communication
Citation: Zhang D, Zhu J, Ma W, Gong M, Wei G, et al. (2015) Urine-Derived Stem Cells for Potential Use in Treatment of Urethral Sphincter Dysfunction. Int J Stem Cell Res 1(2): doi http://dx.doi.org/10.16966/ 2472-6990.105
Copyright: © 2015 Zhang D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access