Introduction
Chronic Myeloid leukemia (CML) is a disorder of the myeloid
lineage [1]. It accounts for almost 15% of all adult leukemias. It results
in uncontrolled and abnormal growth of white blood cells referred to as
blast cells. The disease progresses through three major stages i.e. chronic
phase, accelerated phase and blast crisis phase which is decided on the
number of blasts cells present in the patient’s blood. If a patient is not
treated at an early stage, the disease progresses to the second, third,
accelerated and blast crisis phase respectively [2]. The actual trigger
behind this progression is still not clearly understood. But, in most cases
the reciprocal translocation mutation t[9:22] also known as Philadelphia
chromosome results in a constitutively increase in tyrosine kinase activity
due to this fusion BCR/ABL gene protein [3]. This constitutive expression
of the fusion gene is the central target of this disease. FDA has approved
certain drugs such as Imatinib (ST1571, Glivec from Novartis) which has
now been established as a first line treatment for CML, generating full
cytogenetic response in about majority of the patients in initial stage [4].
Similar drugs with improved mechanism of tyrosine kinase inhibition
(TKI) have come up but none has been able to establish itself as a fully
curative therapy. Most of the TKIs have not been much effective on
patients at advanced stages. Patients with certain point mutations are
resistant to these treatments [5-7]. It is only effective to inhibit the TK
activity but not effective in eradicating the cancer stem cell subpopulations
[8]. At such a condition, Bone Marrow Transplant (BMT) remains as the
only known cure for this disease [9] but, it also has its own limitations.
Finding a suitable donor is one of the major problems in this therapy and
patients also require crucial care to check graft vs. host reactions [10].
Hence, not all of the CML patients can be treated with allogeneic stem cell
therapy, as it requires certain optimal conditions in patient’s body. This has
provoked scientists since long to establish autologous stem cell therapy
(ASCT) as a full curative measure. ASCT could be used for children, older
aged patients and those suffering from advanced form of this disease. It
does not trigger adverse graft reactions and hence can be widely used for
a variety of patients.
Our lab has been working in isolating mesenchymal stem cells from
many hematological malignancies and it was found that the MSCs isolated
in vitro, have shown non-malignant phenotypes or disease free genes [11].
MSCs are now recently looked upon as an alternative to overcome the
problems associated with allogeneic HSCT by using them in combination
with this established therapy [12-14]. We therefore propose to isolate
mesenchymal stem cells from CML positive patient’s peripheral blood
and characterize those using specific molecular markers to understand
its phenotypes and nature. As CML has the characteristic fusion of BCR/
ABL gene, we considered detecting these cells for BCR/ABL transcript
and also profile them for some common oncogenic markers. Apart from
BCR/ABL, we also made use of several other oncogenic markers for
profiling and characterizing these isolated cells to check for their normal
phenotype by immunofluorescence, RT-PCR analysis and anchorage
independent growth assay. If they are found to be normal and free from
any abnormalities, these cells can be potentially useful along with BMT
for better cure of this disease.
Materials and Methods
Sample collection
CML patients’ peripheral blood (10 ml) was collected according to
ethical guidelines of Jaslok hospital and Research Centre, Mumbai, India.
Aliquots of cells were subjected to separation using Ficoll hypaque (Himedia,
India) method. Intermediate layer was aspirated and transferred
to 1.7 ml Eppendorf tubes. Centrifugation was carried out at 4000 rpm
for 10 minutes to obtain pellet which was suspended in 1 ml RPMI media,
having 10% FBS, 0.2% Glutamine, 0.1% Insulin and 1% Pen Strep to
obtain monolayer cell suspension which was cultured in 65 mm Nunc®
petri dishes and incubated overnight at 37°C with 5% CO2 and at 90%
humidity in CO2
incubator. Remaining part of plasma was used for RNA
extraction as described below.
Isolation of mesenchymal stem cells from peripheral blood of CML patient
The cells suspended in above growth media were incubated at 37°C
with 5% CO2 at 90% humidity in CO2 incubator as described above. The adherent cells were washed with 1X PBS and fed with DMEM medium with 10% FBS, 0.2% Glutamine, 0.1% Insulin and 1% Pen Strep. These cells
were observed every day for their growth and photographed using camera
attached to phase contrast microscope. These cells became confluent
within 20 days of culture. After confluency cells were trypsinized with
0.25% PBS-Trypsin for further amplification in several 65 mm flasks,
these cells were then frozen at -85°C till further experimentation. Some
part of these cells were processed for RNA extraction by Trizol® method
and used for gene expression study
Phase contrast microscopy
The growth, proliferation and external morphological features of cells
were inspected using Phase Contrast Microscope (Carl Zeiss Co.) and the
images were documented progressively with the help of attached camera
and TS view software. The observations were made under 20X and 40X
magnifications.
Light microscopy
For observation under compound light microscope, 1 x 105
cells
per ml were grown on sterile cover slips till they reached sub confluent
level. Giemsa staining was carried out to study and observe phenotypic
characteristics of these cells. The cells on the cover slip were washed using
1XPBS and fixed using 50% methanol for 10 minutes. Filtered Giemsa
stain (Fischer Scientific®
) was used for staining the cells for about 10
minutes. The excess stain was washed with distilled water and cover slip
was allowed to air dry before observing under light microscope using 20X
and 40X magnifications.
Immunofluorescence microscopy
For preparing the cells to be used for immunofluorescence, we
cultured 1 × 105
cells per ml on sterile cover slips. On having partial
confluency, the cells were flooded with blocking buffer for about half
an hour and then were fixed using 4% paraformaldehyde. We selected
7 oncogenic antibodies viz. H-Ras, p53, Rb gene, Waf1, p16, EGFR and
Ki-67 which are typically observed in tumor cells. Primary antibody
at 1:10 dilution in blocking buffer was added after washing these cells
with 1X PBS and incubated for 2 hours. FITC labeled Goat anti-mouse
IgG was used as a common Secondary antibody at a 1:100 dilution in
1X PBS under dark condition with all the primary antibodies. It was
added after washing primary antibody with 1X PBS and incubated for 2
hours in dark. The cover slip was washed, dried and inverted on a glass
slide with mounting media, Fluromount (Sigma Aldrich, USA). The
edges of the cover slip were fixed with nail polish and then subjected
to observation under Phase Contrast Microscopy with fluorescent
attachment for FITC excitation and the images were photographed and
documented.
Anchorage independent assay
Mixture of 0.4% soft gel was prepared using 0.8% agarose solution
added in equal volume of double strength RPMI media containing 20%
FBS, 2% Penstrep and 0.4% Glutamine. The solution was cooled upto
40°C and 1.5 ml each was poured on 35 mm Nunc®
culture dishes. Sub confluent culture of cells were trypsinised with 0.25% PBS-Trypsin and
monolayer cells suspension was prepared in the above growth medium
having density of 1 × 104
cells per ml. This suspension was poured on the
soft agar layered culture dishes. Cells were observed and fed with 0.5 ml
RPMI containing 10X FBS, 1% Penstrep and 0.2% glutamine 2-3 times
per week for 2 weeks. After two weeks, we observed the colonies of the
cells appeared on agar plate sand documented them in photographs taken
under phase contrast microscope.
Molecular markers
Total RNA was extracted using Trizol®
(Sigma Aldrich, USA) method.
2 µg of RNA was transcribed to cDNA using Applied Biosystems®
High
Capacity cDNA Kit. We selected various markers for characterization of
this cell line. Primer sequences and PCR conditions for BCR/ABL, CD
105, CD 13, CD 73, CD 34, CD 45, OCT 4, NANOG, SOX 2, LIF, Keratin
18, TNFα, IL 6, Dap Kinase, EGFR and housekeeping gene β Actin have
been previously reported by our lab [15,16]. In addition, we also profiled
3 new markers for the CML cells viz. Bcl-2, cMyc and Notch2 and PCR
conditions were common for all these three genes starting with initial
denaturation at 95°C for 5 minutes, followed by 35 cycles at 94°C for 30
seconds, 60°C for 30 seconds and 72°C for 1minute. Primer sequences of
these genes have been described below (Table 1).
Results
Isolation of adherent cells derived from CML patient’s peripheral blood
We have earlier described in this manuscript about the culturing of
cancer patient derived stem cells. Some of the Ficoll isolated suspension
cells from peripheral blood of CML patient were found to be adhered to the
bottom of the culture dishes within 2-3 days of culture as shown in Figure
1a. These cells then proliferated very quickly and reached confluency
within 20-30 days of culture as shown in Figure 1b. After confluency,
these cells were trypsinised with 0.25% PBS- Trypsin and suspended in
a fresh growth medium & initially plated on Nunc®
35 mm culture dish
for further growth. On attaining confluency, these cells were divided to
create further passages by repeating trypsinization process as described
before. Some cells in the culture were observed to form pluripotent clones
in the succeeding passages as shown in Figure 1c. Thus a stable cell line
was developed and grew in many passages. These cells were then subjected
to phenotypic and genotypic characterization to better understand their
cellular and molecular characteristics.
Giemsa staining
Giemsa staining was carried out for observation of phenotypic
characters of the cells. A network of fiber like structure was observed
around the nucleus connecting it to the cytoplasm. Most of the cells were
very elongated and showed two to three nucleoli in their nucleus. Some
cells were slender and small in size whereas, some were very large and
spreaded. Some cells showed presence of numerous fine filopodia which,
could be noticed after careful observation of the stained cells under high
magnification. Figure 1d shows Giemsa stained picture of these cells.
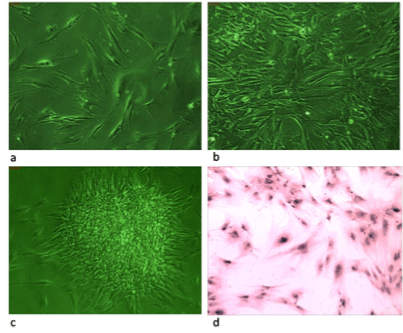
Figure 1a-1d: It shows phase and light microscopic pictures of MSCs
cell line developed from peripheral blood cells of CML patients. Figure 1a shows adherent cells at day 5 of this culture, figure 1b shows well grown confluent cells figure 1c shows pluripotency clone obtained after 10 passages & figure d shows Giemsa stained cells with elongated morphology
Expression of BCR/ABL gene in the isolated stem cells from CML patient
Molecular marker profiling was carried out to find out presence
or absence of certain relevant genes which help in revealing genetic
characters of these cells. BCR/ABL is the fusion gene resulting from
[9:22] translocation. Hence, identifying presence of this gene is of utmost
importance for cells derived from CML patient. Figure 2 shows the
expression of BCR/ABL gene in the patient’s blood plasma cells called as
“in vivo” cells and cells derived in cultures are called as “in vitro” cells at
passage 3 and passage 4. It can be seen that the BCR/ABL gene is present
in blood plasma cells (in vivo cells) but absent in both the cell lines (In
vitro) at passage 3 and 4 indicating that these cells are growing without
BCR/ABL fusion protein. We therefore, designated these cells as BCR/
ABL-ve cells and carried out their further characterization.
Anchorage independent growth assay
Anchorage independent or soft agar assay is mainly used to study in
vitro transformation of the cells growing in culture. Figure 3 shows an
individual assay for BCR/ABL-ve cells. It can be clearly seen that these cells
formed very few anchorage independent colonies by the end of second
week indicating that most of these cells have normal phenotype which
is further confirmed by our studies on cancer molecular markers by RT/
PCR as well as by immunofluorescence Microscopy.
Expression of mesenchymal and hematopoietic markers in BCR/ABL-ve cell line
Stem cells can be characterized for their mesenchymal and hematopoietic
phenotypes using specific molecular markers. Figure 4 shows expression of
mesenchymal and hematopoietic markers in BCR/ABL-ve cells at early
passage P4. The BCR/ABL-ve cells showed presence of CD105, CD13 and
CD73 genes indicating their mesenchymal phenotypes whereas, CML
patient’s in vivo cells showed only presence of CD 105 and absence of CD13
and CD73 genes. The reason for this absence is not known. It was also
seen that BCR/ABL-ve cells mildly expressed CD34 gene at early passage
and diminished after few more passages. These cells did not express
CD45 gene which is another prominent gene marker for hematopoietic
cells. Therefore, absence of CD 45 gene in BCR/ABL-ve cells confirmed
their Mesenchymal phenotype. Thus, we can confirm that the BCR/ABL-ve
cells are mesenchymal stem cells derived from peripheral blood of CML
patients with mild expression of CD34 gene at early passage which is
generally vanished after several passaging of these cells in culture.
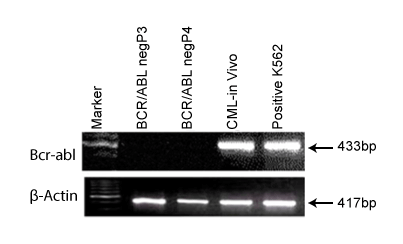
Figure 2: It shows the expression of BCR-ABL gene in “in vivo”cells
but absent in “in vitro” cells of CML patient.
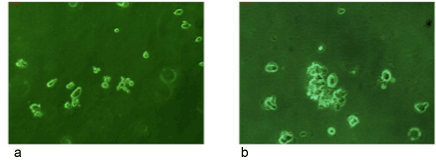
Figure 3: It shows an anchorage independent growth assay (soft
agar assay) for BCR/ABL-ve cells. No proper colonies were found on
soft agar assay even after 2 weeks of plating these cells.
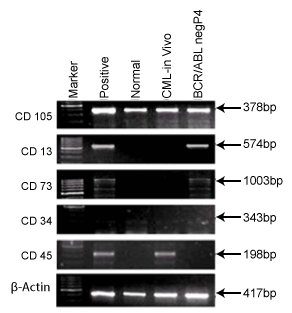
Figure 4: It shows Mesenchymal (CD105, CD13 & CD73) and
Hematopoietic (CD34 & CD45) Markers for BCR/ABL-ve cells and
CML patient’s blood plasma cells i.e in vivo cells.
Expression of pluripotency markers in BCR/ABL-ve cell line
Pluripotency is one of the characteristic properties of stem cells. In
figure 5, we showed the expression of Oct4, Nanog and Sox 2 genes in
BCR/ABL-ve cells and in CML patient’s blood cells (in vivo). It was shown
here that both in vivo cells of CML patient as well as BCR/ABL–ve cell line
showed presence of OCT4 and Nanog genes confirming their pluripotent
nature.
Expression of differentiation markers in BCR/ABL-ve cell line
Differentiation is a stage where the stem cells determine themselves
into a particular phenotype. Figure 6 shows differentiation markers
which reveal presence of LIF and Keratin in both in vivo CML cells and
BCR/ABL-ve cells which indicate that the BCR/ABL–ve cells maintain
undifferentiated and epidermal state in culture as it is in vivo. LIF mainly
helps to keep undifferentiated status whereas; Keratin expression indicates
the epidermal character of these cells.
Expression of cytokines markers in BCR/ABL-ve cell line
Cytokines are prognostic markers for many disease conditions. Figure
7 shows the expression of TNFα and IL6 in both in vivo and BCR/ABL-ve
cells. It was shown that TNFα was expressed in both cell types whereas,
IL6 was only present in BCR/ABL-ve cells and was absent in CML patient’s
in vivo cells.
Expression of various oncogenic markers in BCR/ABL-ve cell line
As we have aimed to use these cells for transplantation purpose along
with BMT, we need to check this BCR/ABL-ve cell line for any oncogenic
abnormalities exist through previous disease cells. To confirm this, we
have chosen specific oncogenic markers such as Dap kinase, Bcl2, Notch,
cMyc and EGFR genes for this study. Figure 8 displays the results of
various oncogenic markers which were used to profile the BCR/ABL-ve
cells at passage 3 (P3) and passage 4 (P4). It was observed that Dap kinase,
Bcl2 and Notch2 genes were present in both in vivo and BCR/ABL-ve cells
whereas, cMyc and EGFR were found to be absent in both in vivo as well
as BCR/ABL-ve cells indicating their normal phenotypes and did not have
any oncogenic abnormality. Thus this BCR/ABL-ve cells can be used for
transplantation program along with BMT process for better cure of this
disease.
Immunofluorescence microscopy localized oncogenic markers in BCR/ABL-ve cell line
Immunofluorescence is a tool used to identify presence and
localization of proteins in the given cells. We have studied localization
of specific oncogenic, tumor suppressor, nucleolus and surface receptor
proteins in BCR/ABL-ve cells to confirm their normal phenotype by
immunofluorescence microscopy
Figure 9A shows the presence of expression of H-Rasoncoprotein in
most of the BCR/ABL-ve cells cytoplasm. Similarly all the tumor suppressor
proteins such as p53, Rb, p16 & p21 were present in almost all these cells.
p53 was observed throughout the cell’s structure and present in both
nuclear as well cytoplasmic region as shown in Figure 9C. Retinoblastoma
protein (Rb) and p16 genes were present in nuclear region only as shown
in Figures 9B and 9E respectively. In p16 staining, nucleolus remained
unstained while rest of nucleus was stained prominently. Whereas, p21
protein was found to be present in some organelle like structure below the
nucleus (Figure 9 D).
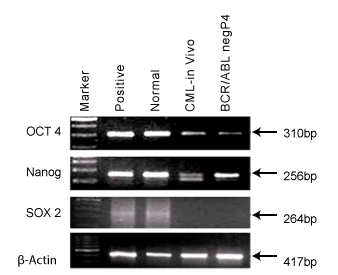
Figure 5: It shows pluripotency makers, OCT4, NANAOG & SOX2 in
BCR/ABL-ve cells and CML patient’s blood cells
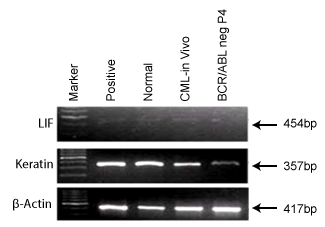
Figure 6: It shows differentiation markers, LIF and Keratin in BCR/
ABL-ve cells and CML patient’s blood cells
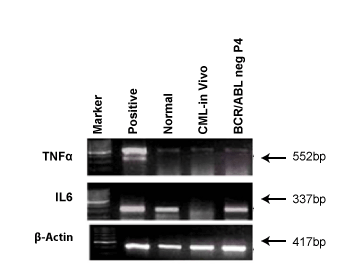
Figure 7: It shows cytokine markers, IL6 and TNFα and Keratin in
BCR/ABL-ve cells and CML patient’s blood cells
A very small percentage of cells showed fluorescence with antigens Ki-
67 and EGFR. Ki-67 was found to be present exclusively in the nucleolus
which has been shown in Figure 10A whereas, EGFR was absent in BCR/
ABL-ve cells as shown in Figure 10B.
So overall immunofluorescence studies confirmed normal phenotypes
of BCR/ABL-ve cell line and thus these cells can be used for transplantation
studies in combination with BMT for cure of this disease in near future.
Discussion
Chronic myeloid leukemia is a progressive disorder of the stem cells,
whose only cure in the blast crisis stage is a BMT which requires HLA
matched allogenic donors for HSCT. In this condition, its availability and
transplant-associated complications make it a vexed procedure. Hence this
disorder along with many others still awaits an ideal cure with minimal
side effects. MSCs are being looked upon as new therapeutic agents for
a number of diseases including hematological malignancies [12-14].
MSCs in co-transplants with umbilical cord stem cells or hematopoietic
stem cells have given positive results. The biggest benefit in choosing
MSCs for autologous or allogeneic transplant is reduction or complete
evasion from graft vs. host reactions. They are also known to improve the
efficiency of hematopoietic transplants. Hence, we channelized our study
to develop MSCs from peripheral blood of CML patient and characterized
them for normal phenotype using various biomarkers by RT/PCT and
Immunofluorescence microscopy.
Our lab at Jaslok hospital and research centre has been working on
isolating, characterizing and culturing of stem cells from many cancer
tumors and hematological malignancies [11]. We considered isolating
stem cells from CML patients and to study their phenotypic characters
and molecular mechanism to see whether, these stem cells are safe enough
to be used for autologous stem cell transplantation in combination with
BMT. We further assessed these stem cells for presence or absence of
BCR/ABL transcript and we found that cells isolated and grown from
CML patients were BCR/ABL-ve cells and therefore we designated these
cells as BCR/ABL-ve cell line. A similar study was carried out by Carrara
et al. [17] and our results are obtained in concordance with them. Upon
establishment of the BCR/ABL-ve cell line and thorough observation of
phenotypic characters through phase contrast microscopy and Giemsa
staining, we could taper down to the idea of presence of mesenchymal
stem cells. To reassert this opinion, we subjected the cells to certain specific
stem cell markers, which confirmed our conjecture. Molecular profiling
of these cells with expression of CD105, CD13, and CD73 affirmed the
mesenchymal character of these cells. OCT4 and NANOG activity
indicate presence of pluripotent character in these cells [15]. Expression
of LIF is indicative of undifferentiated state of these cells whereas, keratin
which is distinctly expressed is used for identifying epithelial nature of
cells [15]. However, expression of CD34 was also found in these cells
whose presence is generally considered as a negative marker for MSCs.
In our study we detected expression of CD34 at early passage cells and
therefore we got weak expression of CD34 in these cells. However, CD45
which is another marker for hematopoietic cell was completely absent in
these cells indicating that BCR/ABL-ve cells are mesenchymal stem cells
only. The CD34 expression seen in BCR/ABL-ve cells may vanish after few
more passage. Diminishing of CD34 gene has been well reported by Lin et
al. [18]. This could be explained with the help of some previous findings
that, peripheral blood derived cells may show presence ofCD34 and also
indicate that they are a part of vasculature. CD34+ MSCs have also been
reported to show numerous filopodia and that, CD34 is present at the tips
of these filopodia for promoting angiogenesis. Observance of numerous
filopodia in some of the cells has been reported in the results above which
probably support the presence of CD34+ MSCs in our culture. Also, weak
presence of CD34 has been reported in early passages of peripheral blood
derived MSCs which rapidly diminishes in the following passages. Since
we have assessed only an early passage, there are chances of observing
mild presence of CD34 [19-21].
significant amount of IL-6 and therefore there is an added advantage if
they are considered for autologous transplantation in combination with
BMT to cure CML. Whereas, TNFα and Bcl-2 have been shown to be
expressed normally indicating normal phenotype of BCR/ABL-ve cells.
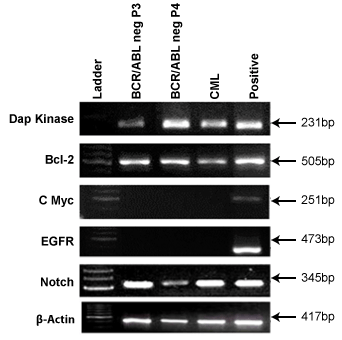
Figure 8: It shows oncogenic markers, DAPK, BCL2, cMYC, EGFR &
NOTCH2 in BCR/ABL-ve cells and CML patient’s blood cells.
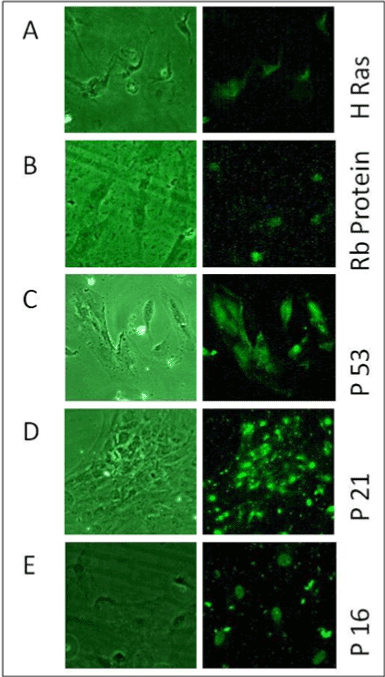
Figure 9: It shows Immunofluorescence microscopy for A) HRas, B)
Rb C) P53, D) P21& E) P16oncoproteins in BCR-ABL–ve cells
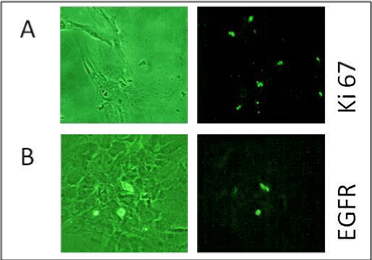
Figure 10: It shows Immunofluorescence microscopy for A) Ki67 & B)
EGFR oncoproteins in BCR-ABL–ve cells
We further used several oncogenic markers and in vivo transplantation
assay to reconfirm normal phenotype of these cells. We selected 5
oncogenic markers viz. Dap Kinase, Bcl-2, cMyc, EFGR & NOTCH2 to
confirm their presence or absence in these cells by RT/PCR analysis. Dap
kinase is known to be a potential tumor suppressor gene and absence of this
gene expedient the growth of cells to form tumor [22]. Our cells showed
presence of Dap kinase gene clearly indicating normal phenotype of BCR/
ABL-ve cells. It is reported that high expression of cMyc causes aggravated
expression of cancerous genes [23]. Our cells showed absence of oncogene
cMyc indicating a normal type again. Deregulation in the signaling of
Notch2 can be a cardinal event in many types of hematological disorders
[24]. Deregulation often leads to reduction in the expression of this gene.
Our BCR/ABL-ve cells showed presence of Notch2, which is again a normal
cell characteristic. EGFR is a cell surface receptor belonging to EGF family.
It has also been reported in certain cases that increased expression of
EGFR, promotes the ability of cancer cells to metastasize and hence many
drugs are focused on down regulation of EGFR to control the cancer [25].
The BCR/ABL-ve cells showed no expression EGFR in these cells indicating
confirmed normal phenotype of BCR/ABL-ve cells. We know that there is
no role of EGFR in causation of CML but we have checked this expression
in MSCs isolated from peripheral blood of CML patients, just to check this
important cancer causing gene in these cells. Recently we have shown that
many of mesenchymal stem cell lines grown in culture are secreting Il-6
cytokines [11]. In our study, BCR/ABL-ve mesenchymal stem cells secrete
significant amount of IL-6 and therefore there is an added advantage if
they are considered for autologous transplantation in combination with
BMT to cure CML. Whereas, TNFα and Bcl-2 have been shown to be
expressed normally indicating normal phenotype of BCR/ABL-ve cells.
We also performed Immunofluorescence to detect presence and
absence of certain oncogenic proteins to confirm normal phenotypes of
BCR/ABL-ve cells. In this study, we observed that very few BCR/ABL-ve
cells showed EGFR protein thus indicating normal phenotype. Similarly
H-Ras, an oncogenic marker was present in almost all of the BCR/ABL-ve cells.
High expression of H-Ras genes along with Waf1 can inhibit proliferation
of K562 cells in vitro. BCR/ABL-ve cells have shown presence of both of
these genes which help in supporting the normal phenotype of these cells
[26,27]. P53 is a well-known tumor suppressor gene and is often down
regulated by BCR/ABL activity [28]. As BCR/ABL is not detected in these
cells, it was expected to observe presence of this protein in cells. As per our
expectations, almost all of these cells showed bright and distinct presence
of p53 throughout the cell and its nucleus. It has also been reported in
some cases that restoration of p53 gene can initiate apoptosis in leukemic
cells as well as increase the proliferation of normal type of cells. We have
also studied Rb protein expression in BCR/ABL-ve cells. As Rb gene is
another tumor suppressor gene which is involved in cancer development.
It has been reported that hematological malignancies might be a result of
dysfunction of Rb gene and its deletion has also been reported in a few
cases [29]. Our study has shown expression of Rb gene in BCR/ABL-ve
cells. Another tumor suppressor gene p16 is tightly controlled by the p53
gene. It promotes senescence in cells and hence can be fatal to many types
of tumor cells [30]. Presence of p16 in our cells indicates normal signaling
of apoptosis, which is another feature of normal cells. We have also
studied Waf1expression which is a gene controlled by p53. It is involved
in anti-apoptosis pathway [31]. It was localized distinctly in an organelle
like structure in the cell’s cytoplasm below the nucleus. So overall these
studies by Immunofluorescence have clearly indicated the normality of
BCR/ABL-ve cell line developed from peripheral blood of CML patients
and thus this cell line can be used as an additional treatment in process of
BMT to cure this disease.
The data collected through these experiments suggest that the cells are
likely normal and do not seem to be obnoxious in any way though derived
from a patient in blast crisis of CML. These results of attaining normal
MSCs are in concordance with previous studies which have reported
isolation of normal MSCs from CML patients [17]. Many studies have
pointed towards the use of MSCs in treating CML. They have been
reported to support haematopoiesis in a study by Zhao Z [31]. They
have also been proven to emolliate GHVD which is one prevalent
problem in HSCT. MSCs when used in co-transplants have shown
better remission in patients supporting their clinical use [20]. The use of
MSCs has been lined with immunomodulatory effects in CML patients
[32]. Koc and Lazarus [12] have explained the strong potentials of the
use of MSCs transplants in many diseases. Recently in an International
conference on Hematology and blood disorders held in US, Farid [14]
has endorsed the idea that MSCs can be used for co-transplantation with
HSCs in treating CML. The use of MSCs co-transplants has shown better
and faster remission in patients as well as hematopoietic repopulation
[12-14]. Hence, MSCs whether allogeneic or autologous, do have strong
therapeutic potentials for treating this disorder in addition to BMT.
There is a need to generate rigorous amount of data with large sample
size and a robust biological marker profile in this area of work to
come to final conclusion in using MSCs derived from CML patient’s
peripheral blood can be used in combination with BMT to cure this
disease without much side effect. We hope that this success will give great
relief to CML suffering patients in near future.
Acknowledgements
Authors wish to thank Management of Jaslok Hospital for funding
this stem cell research project number 491 and Mr. Jacob Texy for his
technical help during this study.











