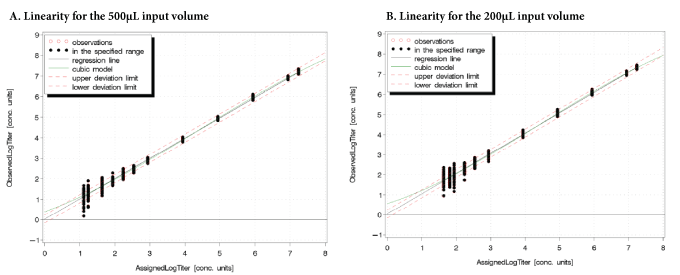
Figure 1: Linearity of the cobas® HIV-1 test for the 500 and 200 µL Sample Volumes


Pirmin Loetscher1 Stefanie Templer1 Britta Seiverth1 Ed Marins2 Christian Simon2 Robert Luo2*
1Roche Diagnostics International Ltd, Rotkreuz, Switzerland*Corresponding author: Robert Luo, MD, MPH, Roche Molecular Systems, Inc., 4300 Hacienda Drive, Pleasanton, CA 94588 USA, Tel: +1 (925) 730-8762; Fax: +1 (925) 225-0875; E-mail: robert.luo.rl1@roche.com
Background and objectives: HIV viral load testing is recommended for all patients on antiretroviral treatment. As viral load testing continues to scale up worldwide, highly sensitive and specific HIV viral load tests that support high throughput testing are needed to provide timely and accurate results. We evaluated the performance characteristics of the new cobas® HIV-1 test for use with the cobas® 6800/8800 system (cobas® HIV-1), and also assessed clinical correlation with the reference standard cobas®AmpliPrep/cobas®TaqMan® HIV-1 Test, version 2.0 (TaqMan®HIV-1 v2.0).
Methods: The limit of detection (LoD), linearity, precision, analytical specificity, diagnostic sensitivity, and specificity of the cobas® HIV-1 test were evaluated. The method comparison to the TaqMan®HIV-1 v2.0 was assessed using Deming regression and Bland-Altman plot analysis, and the mean of the paired viral load difference between the two tests was also calculated using a paired t-test.
Results: The cobas® HIV-1 test demonstrated an LoD of 13.2 copies/mL for HIV-1 M subtype B and a similar LoD for all other HIV-1 subtypes with a linear range of 13 to 1.73 × 107 copies/mL. The assay precision was high with a pooled SD of 0.05 to 0.12 log10 across the linear range. Diagnostic sensitivity using clinical samples was 99.25%, with a specificity of 100%. cobas® HIV-1 demonstrated excellent correlation to TaqMan®HIV-1 v2.0 (R2 =0.98). The overall percentage agreement between the two tests was also high (>95%) with a cutoff of 50 and 200 copies/mL.
Conclusions: The cobas® HIV-1 test is a highly sensitive and specific method for viral load monitoring that has an improved limit of detection, lower sample volume, and increased throughput capability compared to TaqMan®HIV-1 v2.0. The novel cobas® HIV test has excellent correlation with the FDA-approved TaqMan® HIV-1 v2.0 test.
Cobas® HIV-1 test; Cobas® 6800/8800; HIV-1 viral load; TaqMan®HIV-1 v2.0
In July 2013, the World Health Organization recommended HIV viral load testing for monitoring all patients on antiretroviral therapy [1]. Following this recommendation, UNAIDS released new targets for 2020, where 90% of people living with HIV know their status, 90% of those diagnosed with HIV are on antiretroviral treatment, and 90% of those on treatment have suppressed viral loads [2]. In addition to treatment monitoring, viral load testing is also widely used to evaluate newly diagnosed patients, inform treatment decisions, and guide the management of pregnant women with HIV to prevent mother-to-child transmission [3-5]. With approximately 37 to 39 million people now living with HIV around the world [6,7], quantitative viral load tests that are accurate, reliable, and capable of high throughput testing are needed to help meet these ambitious goals and put an end to the AIDS epidemic.
The cobas® HIV-1 test for use on the cobas® 6800/8800 systems (cobas® HIV-1 test) has been developed by Roche Molecular Systems, Inc. (Pleasanton, CA). This is an in vitro quantitative nucleic acid test that employs a dual-target approach to simultaneously amplify and detect both the gag and ltr regions of the HIV-1 genome, which was first introduced for viral load monitoring by the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1, v2.0 Test (TaqMan®HIV-1 v2.0) [8-10]. The test runs on the cobas® 6800 and 8800 systems, which are capable of fully automated testing of up to 384 samples and 960 samples in an 8-hour shift, respectively, and require 650 µL of plasma per patient, of which 500 µL is further processed [11]. The objectives of the current study were to evaluate the performance characteristics of the cobas® HIV-1 test for the detection and quantification of HIV-1 viral load in the plasma of HIV-infected individuals as well as to compare the results of clinical specimens tested with cobas® HIV-1 test to the results obtained with the TaqMan®HIV-1 v2.0 as a reference standard. In addition, the performance of the cobas® HIV-1 test was also compared with different sample input volumes (200 µL and 500 µL), systems (cobas®6800 and cobas®8800), and tubes (with and without gel separators).
The limit of detection (LoD) of the cobas® HIV-1 test was determined by testing serial dilutions of the 2nd HIV-1 World Health Organization international standard (2nd WHO-IS, NIBSC code: 97/650; DH- 268-057C, HIV-1 Group M Subtype B) [12] in negative pooled ethylenediaminetetraacetic acid (EDTA) plasma. Three independent dilution series consisting of 5 concentration levels and a blank were prepared for each process input volume (200 µL and 500 µL) to obtain 63 replicates per level that were tested with multiple reagent lots, systems, and operators over 3 days. The resulting data were analyzed to identify the PROBIT value and 95% hit rate. The LoD was also verified for the following HIV-1 subtypes: Group M Subtypes A, C, D, F, G, H, CRF01_ AE and CRF02_AG, Group O, and Group N using serial dilutions of individual subtype intermediate stock solution in HIV-1 negative EDTA plasma to prepare a testing panel of 3 concentration levels for each input volume.
A linearity panel with 12 panel members for the 500 µL sample input volume and 11 panel members for the 200 µL sample input volume was prepared in HIV-1 negative pooled EDTA plasma using a high-titer HIV- 1 Group M subtype B cell culture supernatant. A total of 48 replicates per concentration level for each panel were tested by 3 operators using 3 reagent lots and 3 instruments over 12 days. All titers were calculated and converted into log10 titer. Linearity was assessed by comparing deviations of predicted values of the linear fit to the predicted values of best-fitting higher order model (according to the significance of 2nd and 3rd order effects) at each measured concentration level according to the methodology defined in the Clinical & Laboratory Standard Institute (CLSI) guideline EP06-A [13]. The maximum deviation between the linear regression and the better fitting non-linear regression was required to be ≤ 0.2 log10 for both sample input volumes. The claimed linear range was also verified for other subtypes using a 7 member dilution panel from cell culture supernatants of HIV-1 Group M subtypes A, C, D, F, G, H, CRF01- AE, and CRF02-AG, HIV-1 Group N, and HIV-1 Group O. Dilutions of the cell culture stocks to approximately 20 copies/mL to 1.00E+07 copies/ mL were tested in 14 replicates using two reagent lots.
Assay precision was calculated using data obtained from the linearity study with an 8-member serial dilution panel of HIV-1 Group M subtype B cell culture supernatant (MVP899) with concentration levels ranging from 15 copies/mL to 2.0E+07 copies/mL (nominal titer). Each panel was tested in 16 replicates across 3 reagent lots for a total of 48 replicates per concentration level per panel. The panels were tested using 3 instruments by 3 operators over 12 days. The precision was computed as per the CLSI guideline EP5-A2 [14].
The analytical specificity of the cobas® HIV-1 test was evaluated by testing for cross-reactivity with 23 different microorganisms. Interference was analyzed by spiking HIV-1 negative EDTA plasma samples with potential endogenous interferents, 45 commercially available drugs that could be administered to HIV-1 infected patients, and clinical samples diagnosed positive for autoimmune syndromes.
The clinical specificity was calculated by testing six hundred HIV negative EDTA plasma samples from individual donors using two lots of cobas® HIV-1 reagents.
Plasma samples from 265 de-identified HIV-1 infected patients with detectable HIV-1 titers on TaqMan®HIV-1 v2.0 testing were tested using two lots of cobas® HIV-1 reagents.
The cobas® HIV-1 test on the cobas®6800 System (cobas® 6800 HIV- 1 test) was evaluated by testing frozen samples (nominal viral load ≥ 20 copies/mL) in EDTA plasma with viral loads spanning the linear range of the test and the results obtained were compared with those obtained with the TaqMan®HIV-1 v2.0 test using the guidelines published in CLSI EP09-A2 [15].The samples were from HIV-1 infected patients and were collected under site-specific Institutional Review Board approved documents for each site.
All testing was performed according to the manufacturer’s instructions as described in the respective package insert [16,17]. Results are reported in copies/mL and are traceable to the WHO 2nd International Standard for HIV-1 RNA (NIBSC 97/650) [12], with one International Unit (IU)/mL being equivalent to 0.6 copies/mL on cobas® HIV-1.
All analyses were performed using SAS® software (SAS/STAT software, version 9.3, SAS Institute Inc. Cary, NC, USA) [18]. Comparisons were made using a) Deming regression analyses and b) scatter and BlandAltman plots. The mean paired difference between the cobas® HIV-1 test and the TaqMan®HIV-1 v2.0 at medically relevant thresholds and the associated 95% confidence intervals (CI) were calculated using a paired t-test.
Concordance analysis between paired cobas®6800 HIV-1 test results and TaqMan®HIV-1 v2.0 results was also assessed. The overall percent agreement (OPA) between the two assays was calculated along with p value (calculated using McNemar’s test) at cutoffs: 200 copies/mL and 50 copies/mL.
The calculated viral load titers obtained from paired specimens used in the clinical methods comparison analysis with cobas® HIV-1were compared between the two sample input volumes (200 µL and 500 µL), systems (cobas® 6800 and cobas® 8800), and tubes (plasma preparation tubes [PPT] with gel separators and conventional EDTA plasma tubes without gel separators). The mean paired difference and associated 95% CIs were calculated for each comparison using a paired t-test.
The cross contamination rate for cobas®6800 HIV-1 test was determined by testing 240 replicates of an HIV negative human EDTA-plasma sample and 225 replicates of a high titer HIV-1 sample at 4.00E+06 copies/mL were tested. In total, five runs were performed with positive and negative samples in a checkerboard configuration.
The LoD for the cobas® HIV test by PROBIT analysis was 13.2 copies/ mL (22.0 IU/mL) for the 500 µL sample input volume and 35.5 copies/mL (59.2 IU/mL) for the 200 µL sample input volume (Table 1). Data were consistent for each lot with a tight 95% CI of 11.4 to 15.9 copies/mL for the 500 µL sample input volume and 30.8 to 43.2 copies/mL for the 200 µL sample input volume.
Volume |
Hit rate ≥ 95% |
95% LoD PROBIT |
95% lower confidence limit |
95% upper confidence limit |
500 µL |
20.0 copies/mL |
13.2 copies/mL |
11.4 copies/mL |
15.9 copies/mL |
33.3 IU/mL |
22.0 IU/mL |
19.0 IU/mL |
26.5 IU/mL |
|
200 µL |
50.0 copies/mL |
35.5 copies/mL |
30.8 copies/mL |
43.2 copies/mL |
83.3 IU/mL |
59.2 IU/mL |
51.3 IU/mL |
72.0 IU/mL |
Table 1: Summary of LoD in EDTA plasma for the 500 µL and 200 µL sample input volumes for HIV-1 Group M Subtype B using the cobas®HIV-1 test
EDTA: Ethylenediaminetetraacetic acid; HIV: Human Immunodeficiency Virus; LoD: Limit of DetectionThe LoD was similar for all other HIV-1 subtypes tested in EDTA plasma for the 500 µL and 200 µL sample input volumes (Table 2). Hit rate analysis demonstrated a positivity rate of greater than 95% for all Group M subtypes and Group N at 20 copies/mL. For Group O, the 95% CI at 20 copies/mL included 95%, suggesting no statistically significant difference between the observed hit rate of 90.5% and a 95% hit rate.
Genotype |
Conc. level |
500 µL |
200 µL |
||
Hit rate |
95% CI |
Hit rate |
95% CI |
||
HIV-1M A |
0.5 × LoD |
93.7% |
84.5%, 98.2% |
85.7% |
74.6%, 93.3% |
1.0 × LoD |
100.0% |
94.3%, 100.0% |
95.2% |
86.7%, 99.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1M C |
0.5 × LoD |
81.0% |
69.1%, 89.8% |
79.4% |
67.3%, 88.5% |
1.0 × LoD |
96.8% |
89.0% 99.6% |
98.4% |
91.5%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1M D |
0.5 × LoD |
76.2% |
63.8%, 86.0% |
81.0% |
69.1%, 89.8% |
1.0 × LoD |
96.8% |
88.8%, 99.6% |
100.0% |
94.3%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1M F |
0.5 × LoD |
93.7% |
84.5%, 98.2% |
88.9% |
78.4%, 95.4% |
1.0 × LoD |
100.0% |
94.3%, 100.0% |
98.4% |
91.5%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1M G |
0.5 × LoD |
85.7% |
74.6%, 93.3% |
82.5% |
70.9%, 90.9% |
1.0 × LoD |
100.0% |
94.3%, 100.0% |
98.4% |
91.5%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1M H |
0.5 × LoD |
82.5% |
70.9%, 90.9% |
96.8% |
89.0%, 99.6% |
1.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
CRF01_AE |
0.5 × LoD |
82.5% |
70.9%, 90.9% |
84.1% |
72.7%, 92.1% |
1.0 × LoD |
98.4% |
91.5%, 100.0% |
90.5% |
80.4%, 96.4% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
CRF02_AG |
0.5 × LoD |
88.9% |
78.4%, 95.4% |
77.8% |
65.5%, 87.3% |
1.0 × LoD |
98.4% |
91.5%, 100.0% |
100.0% |
94.3%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1O |
0.5 × LoD |
77.8% |
65.5%, 87.3% |
69.8% |
57.0%, 80.8% |
1.0 × LoD |
90.5% |
80.4%, 96.4% |
88.9% |
78.4%, 95.4% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
HIV-1N |
0.5 × LoD |
90.5% |
80.4%, 96.4% |
87.3% |
76.5%, 94.4% |
1.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
2.0 × LoD |
100.0% |
94.3%, 100.0% |
100.0% |
94.3%, 100.0% |
|
Table 2: Summary of hit rates for HIV-1 subtypes using the cobas®HIV-1 test for the 500 µL and 200 µL sample input volumes
The acceptance criteria are met for each subtype when the hit rate for either of the tested levels, and all of the above levels tested, is equal to or greater than 90.5% (n=63) or 90.3% (n=62).
CI: Confidence Interval; Conc: Concentration; HIV: Human Immunodeficiency Virus; LoD: Limit of Detection.
The linear range of the cobas® HIV-1 test was 13 to 1.73E+07 copies/ mL (log10 1.11–7.24) for the 500 µL process input volume and 43.4 to 1.73E+07 copies/mL for the 200 µL process input volume. The claimed linear range of the test is 20 to 1.0E + 07 copies/mL (log101.30–7.00) with the 500 µL process input volume. The maximum deviation between the linear regression and the better fitting non-linear regression was equal to or less than 0.2 log10. Across the entire linear range, the accuracy of the test was within ± 0.20 log10 (Figure 1). For other subtypes also, the maximum deviation between the linear regression and the better fitting non-linear regression was equal to or less than 0.2 log10.

Figure 1: Linearity of the cobas® HIV-1 test for the 500 and 200 µL Sample Volumes
The LLOQ was determined to be equal to LoD for both sample input volumes.
The total precision estimated as the standard deviation (SD) of log10 titers was comparable across all lots and reproducible in both input volumes (200 µL and 500 µL). The cobas® HIV-1 test showed high precision; the pooled SD of log10 titers was within ± 0.12 log10 across a concentration range of 86.7 copies/mL to 8.67E+06 copies/mL for the 500 µL input volume (table 3) within ± 0.08 log10 across a concentration range of 8.67E+03 copies/mL to 8.67E+06 copies/mL for the 200 µL input volume (Table 3). Variance component analysis revealed that day and within run for the 500 µL input volume and kit lot and within run for the 200 µL input volume had the largest contribution to the total precision variance.
Panel member |
Assigned concentration |
|
Mean observed (log titer) |
|
Total precision as SD (log ) |
Pooled SD |
||
|
TPV Lot 2 |
TPV Lot 3 |
TPV Lot 1 |
TPV Lot 2 |
TPV Lot 3 |
|||
A. 500 µL input volume |
||||||||
PM02 |
8.67E+06 |
6.92 |
6.92 |
6.91 |
0.04 |
0.06 |
0.03 |
0.05 |
PM03 |
8.67E+05 |
6.00 |
5.97 |
5.98 |
0.06 |
0.05 |
0.04 |
0.05 |
PM04 |
8.67E+04 |
4.97 |
4.95 |
4.92 |
0.05 |
0.07 |
0.04 |
0.05 |
PM05 |
8.67E+03 |
3.94 |
3.90 |
3.88 |
0.06 |
0.06 |
0.04 |
0.05 |
PM06 |
8.67E+02 |
2.91 |
2.87 |
2.85 |
0.07 |
0.06 |
0.07 |
0.07 |
PM07 |
3.47E+02 |
2.47 |
2.47 |
2.44 |
0.09 |
0.10 |
0.09 |
0.09 |
PM08 |
1.73E+02 |
2.20 |
2.19 |
2.16 |
0.11 |
0.08 |
0.14 |
0.11 |
PM09 |
8.67E+01 |
1.92 |
1.92 |
1.92 |
0.15 |
0.11 |
0.10 |
0.12 |
B. 200 µL input volume |
||||||||
PM02 |
8.67E+06 |
7.09 |
7.03 |
7.04 |
0.04 |
0.05 |
0.04 |
0.04 |
PM03 |
8.67E+05 |
6.12 |
6.06 |
6.07 |
0.07 |
0.05 |
0.05 |
0.06 |
PM04 |
8.67E+04 |
5.09 |
5.07 |
5.04 |
0.07 |
0.07 |
0.06 |
0.07 |
PM05 |
8.67E+03 |
4.04 |
4.00 |
3.96 |
0.08 |
0.08 |
0.06 |
0.08 |
PM06 |
8.67E+02 |
3.03 |
2.95 |
2.91 |
0.12 |
0.12 |
0.08 |
0.11 |
PM07 |
3.47E+02 |
2.64 |
2.57 |
2.51 |
0.11 |
0.13 |
0.09 |
0.11 |
PM08 |
1.73E+02 |
2.30 |
2.29 |
2.26 |
0.20 |
0.12 |
0.15 |
0.16 |
Table 3: Summary of total precision as SD of log10 titer results for the cobas® HIV-1 test
PM: Panel Member; SD: Standard Deviation; TPV: Technical Performance Verification
All 600 clinical plasma samples tested negative for HIV-1 RNA, resulting in a diagnostic specificity of 100% (one-sided lower 95% CI: ≥ 99.5%). Analytical specificity was evaluated by testing 28 cultured microorganisms at 1.00E+0.6 particles, copies, IU, genome equivalents, or colony forming units/mL, including 25 viral isolates. Negative results were obtained for all samples without HIV-1 RNA, and positive results were obtained on all samples with HIV-1 RNA. Mean log10 titers of positive HIV-1 samples were all within ± 0.16 log10 of the mean log10 titers of spiked controls with the same amount of HIV-1 RNA but no microorganisms. When tested at the medically relevant concentrations, none of the common endogenous and exogenous interferents interfered with the performance of the cobas® HIV-1 test. The mean difference of the observed log10 titers for the HIV- 1 positive spiked samples to the mean log10 titer of the respective spike control was within ± 0.30 log10.
A valid quantitative result was achieved in 263 of the 265 HIV-1 clinical samples, resulting in a sensitivity of 99.25%. An additional external validation study was performed to assess sensitivity on clinical samples at an external site. Remainders of 109 plasma samples from HIV-1 patients with detectable HIV-1 titers were tested, and positive valid results were obtained on all samples, resulting in a sensitivity of 100% (109/109).
Out of 410 subjects enrolled in the study, all were eligible for concordance analysis and 305 subjects had overlapping linear range for comparison between the cobas® 6800 HIV-1 test and the TaqMan®HIV-1 v2.0 test.
Table 4 shows the summary of demographic and clinical characteristics of the enrolled patients. The mean age was 41.8 years and the majority of patients (78.3%) were male. The most common HIV-1 subtype in the enrolled patients was subtype B (52.2%).
Characteristics |
All patients (N=410) |
Age (years) |
|
Mean (SD) |
41.8 (11) |
Median |
43 |
Range |
19 to 72 |
Gender |
|
Male |
321 (78.3%) |
Female |
89 (21.7%) |
Race |
|
Asian |
5 (1.2%) |
Black |
163 (39.8%) |
Latino |
17 (4.1%) |
White |
94 (22.9%) |
Other |
91 (22.2%) |
Unknown |
40 (9.8%) |
HIV subtype |
|
A |
4 (1.0%) |
B |
214 (52.2%) |
C |
2 (0.5%) |
D |
7 (1.7%) |
G |
2 (0.5%) |
N |
5 (1.2%) |
CRF01_AE |
4 (1.0%) |
B/D |
1 (0.2%) |
CRF02_AG |
2 (0.5%) |
Unknown |
169 (41.2%) |
Antiviral medication |
|
Yes |
208 (50.7%) |
No |
137 (33.4%) |
Unknown |
65 (15.9%) |
CD4 Cell Count (cells/µL) |
|
N |
391 |
Mean (SD) |
438.1 (267.7) |
Median |
401 |
Range |
0 to 1548 |
CD8 Cell Count (cells/µL) |
|
N |
283 |
Mean (SD) |
924.4 (451.2) |
Median |
838 |
Range |
150 to 2446 |
Table 4: Demographic and clinical characteristics of the patients enrolled in clinical method comparison analysis
HIV: Human Immunodeficiency Virus; SD: Standard Deviation.
Deming regression analysis demonstrated high correlation (R2 =0.98 [95% CI of intercept: -0.180 to -0.014 and 95% CI of slope: 1.036 to 1.075]) among samples detected by the cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 tests within the overlapping linear range (Figure 2 and Table 5). When plotted, most points were above the line of unity (Y=X) indicating that the cobas® 6800 HIV-1 test returned higher titers than the TaqMan®HIV-1 v2.0 (Figure 2).
Figure 2: Deming regression analysis ofcobas®6800 HIV-1 test vs TaqMan® HIV-1 v2.0, EDTA plasma samples (log10 copies/mL)
CI: confidence interval
Number of paired samples = 305 |
||||
Parameter |
Parameter estimate |
Standard error |
95% CI |
R2 |
Intercept |
-0.097 |
0.042 |
-0.180 to -0.014 |
0.98 |
Slope |
1.055 |
0.010 |
1.036 to 1.075 |
|
Table 5: Parameter estimates of Deming regression between viral loads of cobas®6800 HIV-1 test and TaqMan® HIV-1 v2.0
Note: Samples tested using cobas® 6800 were collected in EDTA plasma tubes and tested with the 500 µL sample input volume. This table only includes paired results that were each within the overlapping linear range of both assays (2.0 E+01 to 1.0 E+07 copies/mL) CI: Confidence Interval.
The mean of the paired viral load difference between the cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 test results across the entire linear range was 0.112 log10 copies/ml (Table 6). The mean difference of log10 titers was ≤ 0.101 log10 copies/mL at almost all medically relevant decision intervals with a difference of 0.222 log10 copies/mL at ≥ 100,000 copies/mL (Table 6).
Figure 3 shows the bias plot of the viral load difference between cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 (Y-axis) versus theTaqMan®HIV-1 v2.0 viral load (X-axis). Most differences were above zero indicating that the cobas® 6800 results returned higher titers than onTaqMan®HIV-1 v2.0.
Figure 3: Bias plot of viral load difference cobas®HIV-1 6800 test and TaqMan® HIV-1 v2.0versus TaqMan® HIV-1 v2.0 viral load result (log10 copies/mL)
Figure 4 shows the Bland-Altman plot of the viral load difference between cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 (Y-axis) versus the average of the cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 viral loads (X-axis). The majority of the data points were between the thresholds of ± 0.5 log10 copies/mL throughout the linear range.
Figure 4: Bland-Altman bias plot of viral load difference versus average viral load between cobas®HIV-1 6800 test and TaqMan® HIV-1 v2.0 (log10 copies/mL)
CI: confidence interval
Concordance analysis between the cobas® 6800 HIV-1 and TaqMan®HIV-1 v2.0 tests demonstrated that the OPA at a cut-off of 200 copies/mL was 96.8% (397/410; 95% CI: 94.6% to 98.3%) and at a cutoff of 50 copies/mL was 95.4% (391/410; 95% CI: 92.9% to 97.2%) (Table 7). The proportion of results <200 copies/mL on the cobas® 6800 was not statistically significantly different from that of TaqMan®HIV-1 v2.0 (p value=0.581). However, there was a statistically significant difference between the proportion of results <50 copies/mL on the cobas® 6800 and TaqMan®HIV-1 v2.0 (p=0.0192).
The mean difference between viral loads of samples tested with the 200 µL and 500 µL sample input volumes was 0.094 log10 copies/mL (95% CI: 0.067 to 0.121) on the cobas® 6800 HIV-1 system and 0.080 log10 copies/ mL (95% CI: 0.056, 0.105) on the cobas® 8800 HIV-1 system. Higher titers were returned using the 200 µL sample input volume.
The estimate of systemic bias calculated using the mean of the paired viral load difference between the cobas® 6800 and 8800 systems was very low for both sample input volumes (0.011 with a 95% CI of -0.014 to 0.036 for 200 µL and -0.001 with a 95% CI of -0.024 to 0.022 for 500 µL).
The mean log10 titer differences on 42 paired samples was 0.026 log10 (95% CI -0.029 to 0.081 log10). Higher titers were returned with the plasma samples in PPT than in EDTA plasma tubes; the results showed that the 95% CI of the mean of the paired viral loads was quite tight and included “0”.
All 240 replicates of the negative sample were negative, resulting in a cross contamination rate of 0% (95% CI: 0% to 1.5%).
The cobas® HIV-1 test provides a highly sensitive and specific method for measuring HIV-1 RNA viral loads in a precise and accurate manner. Excellent correlation is seen compared to the TaqMan®HIV-1 v2.0 test and results were not affected by any potential endogenous or exogenous interfering substances tested.
Based on PROBIT analysis, the LoD of the cobas® HIV-1 test for the prominent HIV-1 subtype (Group M subtype B) was found to be 13.2 copies/mL using a sample input volume of 500 µL. Data were consistent for each lot with a tight 95% CI of 11.4 to 15.9 copies/mL (19.0 to 26.5 IU/mL). The LoD of this novel test was determined to be comparable to TaqMan®HIV-1 v2.0 (20.0 copies/mL) and better than the LoD determined for the Abbott RealTimeHIV-1 assay (40 copies/mL) [19-21]. The LoDs for all other tested HIV-1 subtypes (HIV-1 Group M subtypes A, C, D, F, G, H, CRF01-AE, and CRF02-AG, HIV-1 Group N, and HIV-1 Group O) were similar to that for HIV-1 subtype B.
In the current analysis, the cobas®HIV-1 test was shown to have broad linear range of 13 copies/mL to 1.73 × 107 copies/mL with HIV-1 Group M subtype B material which is traceable to the 2nd WHO international standard. The observed linear range meets the acceptance criteria for the assay (20 copies/mL to 1 × 107 copies/mL) and is also comparable to or better than the linear range of other FDA-approved assays (the TaqMan®HIV-1 v2.0 test: 20 copies/mL to 1 × 107 copies/mL and the Abbott RealTime HIV-1 assay: 40 copies/mL to 1 × 107 copies.mL) [22]. The precision of the cobas®HIV-1 test calculated over 12 days, on three cobas® 6800 instruments and with three reagent lots was determined to be high with a pooled SD in the range of 0.05 to 0.10 log10 titer across a concentration range 86.7 copies/mL to 8.67 × 106 copies/mL.
The cobas® HIV-1 test displayed excellent diagnostic and analytical specificity of 100%. There was no interference from 23 potentially interfering microorganisms, common endogenous substances, and drug compounds commonly administered to HIV-1 infected patients in the results obtained. Thus, it can be interpreted the test will not provide false positive results due to non-specific target amplification, and is highly specific to HIV-1 viremia. The cobas®HIV-1 test demonstrated high sensitivity of 99.25% to 100.
The cobas®6800 HIV-1 test was compared to the highly sensitive and accurate FDA–approved, TaqMan®HIV-1 v2.0 test (reference standard) [23,24]. The comparisons showed high correlation between the two assays (R2 =0.98), with a mean paired viral load difference of 0.112 log10 copies/ mL (95% CI: 0.086, 0.137). The concordance between the two tests was also high with the OPA>95% at cut-offs of 50 copies/mL and 200 copies/mL. The proportion of results <200 copies/mL was not statistically significantly different between the two tests (p value: NS). The performance of the cobas® HIV-1 test was compared to TaqMan®HIV-1 v2.0 in another study by testing in duplicate 251 matched plasma samples from patients known to be infected with HIV-1 along with 16 diluted cell culture supernatants. Deming regression analysis demonstrated an R2 value of 0.98, with samples spanning the linear range of both assays and comprising a mix of subtypes [16]. A similar comparison was performed with the Abbott RealTime HIV-1 assay on 224 total samples. Deming regression analysis demonstrated an R2 value of 0.97. These results demonstrate that the three assays have equivalent performance and are commutable.
The mean of the paired viral load difference between the cobas®HIV-1 test and TaqMan®HIV-1 v2.0 was even smaller at the medically relevant decision intervals of 50 to <200, 200 to <400, and 400 to <1,000 copies/ mL (-0.004 to 0.032 log10 copies/mL), with all 95% CIs including “0”. It is observed that the results around the 200 copies/mL level are the most critical to determining if HIV-infected patients are experiencing virologic failure while on treatment [3]. In the current study, there was no statistically significant difference between the cobas®HIV-1 test and TaqMan® v2.0 results for this level.
The study also demonstrated that there were no clinically significant differences between paired viral load measurements from 2 different sample input volumes (200 µL versus 500 µL) and between the cobas®6800 and 8800 systems.
The new cobas® HIV-1 assay requires only 650 µL of plasma, compared to a 1000 µL sample volume on the TaqMan®HIV-1 v2.0 test [25]. Additionally, both lavender top EDTA plasma tube and BD PPT tubes can be used with equivalent results, simplifying sample collection and specimen transport across many settings.
As viral load testing expands to millions more people over the next few years, laboratories will need high throughput HIV viral load testing solutions. The cobas® HIV-1 test runs on the cobas® 6800 and 8800 systems, which are capable of fully automated testing of up to 384 samples and 960 samples in an 8-hour shift, respectively [16]. The high throughput nature of the platforms should enable more cost effective testing, with less staff time per sample needed. Additionally, the systems allow for automated mixed batching with other assays, enabling accurate and timely results for laboratories doing a variety of molecular tests.
In conclusion, the cobas® HIV-1 test is a state-of-the-art molecular test that uses a dual-target approach against both the gag and ltr regions of the HIV genome to provide accurate, high throughput viral load monitoring with highly conserved genetic targets that are not susceptible to mutation pressure from current antiretroviral therapies. The cobas® HIV-1 test provides a highly sensitive and specific method for measuring HIV-1 RNA viral loads in a precise and accurate manner. Excellent correlation was seen compared to the TaqMan®HIV-1 v2.0 test and results were not affected by any potential endogenous or exogenous interfering substances tested. Overall, the novel cobas® HIV-1 test on cobas® 6800/8800 system provides improved performance characteristics and can provide an important tool for the clinical management of HIV.
This study was supported by Roche Molecular Systems, Inc. All authors are employees of Roche. The cobas® HIV-1 is not available in all markets.
Download Provisional PDF Here
Article Type: Research Article
Citation: Loetscher P, Templer S, Seiverth B, Marins E, Simon C, et al. (2017) Performance Evaluation of Cobas® HIV-1, a Quantitative Nucleic Acid Test for Use on the Cobas® 6800/8800 Systems. J HIV AIDS 3(1): doi: http://dx.doi.org/10.16966/2380-5536.135
Copyright: © 2017 Loetscher P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access