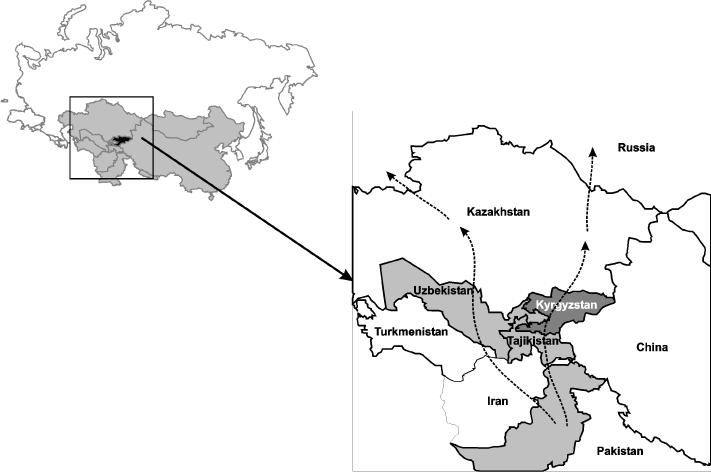
Figure 1: A map of Kyrgyzstan and its bordering countries in central Asia. The dashed lines show some common drug trafficking routes.

Vita Laga1 Ilya Lapovok1 Elena Kazennova1 Aikul Ismailova2 Nurgul Beisheeva2 Nazgul Asybalieva2 Nataliya Glushchenko1 Marina Bobkova1*
1Ivanovsky Institute of Virology, Moscow, Russia*Corresponding author: Marina Bobkova, Laboratory of T-lymphotropic Viruses, Ivanovsky Institute of Virology, 123098 Moscow, Russia, Gamaleya 16, Tel: +7 (499) 190 30 63; Fax: +7 (499) 190 30 63; E-mail: mrbobkova@mail.ru
To evaluate HIV-1 variability and drug resistance, a cross-sectional study involving 57 naïve patients was carried out in Kyrgyzstan, Central Asia, between 2009 and 2010. Most of the patients were men (77.2%), and most of them were injecting drug users (97.7%). To analyze the HIV-1 genetic variability, DNA sequencing in both the PR-RT and gag genome regions was performed.
The study identified complex HIV-1 molecular epidemiological patterns in Kyrgyzstan with the co-circulation of multiple subtypes, including currently circulating and unique recombinant forms. CRF02_AG was the predominant genetic form (45.6%; 26/57), and the second-most prevalent HIV-1 genetic variant was subtype A1 (IDU-A) (40.4%; 23/57). The phylogenetic analysis results indicate a link between the HIV epidemic in Kyrgyzstan and Russia and Ukraine on the one hand and other Central Asian FSU countries on the other hand. The simultaneous analysis of pol and gag regions allowed the identification of seven unique recombinant forms (12.3%) formed by two major variants (CRF02_AG/A1), each having a specific PR-RT structure. The analysis of drug resistance mutations found NRTI mutations M184I and K65R and NNRTI mutations Y181C, K103N, and G190S in four sequences; no mutations in the PR region were detected.
The representativeness of this study was limited both in terms of collection size and in terms of the incomplete representation of different population groups. Further studies are needed to better understand the evolution of HIV-1 genetic variants and drug resistance in Kyrgyzstan and Central Asia.
HIV-1; Subtype; Recombinant; Variability; Kyrgyzstan
Kyrgyzstan together with Kazakhstan, Tajikistan, Turkmenistan and Uzbekistan (Figure 1) comprise the former USSR (FSU) Central Asian countries, which are among the countries with the highest growth rate of the HIV epidemic [1,2]. Although the overall prevalence of HIV among the general population remains low (117.9, 86.8 and 77.9 per 100,000 in Kazakhstan, Tajikistan, and Kyrgyzstan, respectively), the reliability of these figures may be insufficient; indeed, according to experts, this number may be several times higher [3,4]. The number of people newly diagnosed with HIV in the Central Asian region in 2011 was 14 times higher than in 2000 [1]. Thus, without effective programs, HIV is expected to spread rapidly in the region as a whole and in Kyrgyzstan in particular.
The Kyrgyz Republic is a low-income, mountainous, and predominantly agrarian country with more than 64% of its 5.3 million populations residing in rural areas. Drug use is a historical tradition of this region in the form of per os use of opium and poppy tea, but during the last two decades, a significant shift in drug use patterns among drug abusers toward a marked increase in heroin injection has been observed, with major risks for HIV acquisition [5]. It is reasonably believed that such a significant increase in injecting drug use coincided with the collapse of the USSR in 1991 [6-8], as well as with the growth of poverty, on the one hand and with the geographical location of Kyrgyzstan between Afghanistan and Russia on the other hand. As seen in Figure 1, the country is located alongside one of the major heroin trafficking routes out of Afghanistan, including Russia with the largest market for Afghan heroin [9]. One of the predictable consequences of this was an increase in HIV infection numbers. During the period from 2000 to 2010, the prevalence of HIV infection in the Kyrgyz Republic increased 55-fold (from 1.1 to 60.7 per 100 thousand populations) [10].
By the beginning of this period, a massive epidemic of HIV infection had already been developing in most FSU countries. The epidemic among injecting drug users (IDUs) started in Ukraine in the mid-1990s and was caused by two single introduction events involving the A1 genetic variant, likely originating from West Africa (IDU-A), and the subtype B variant (IDU-B), the origin of which remains unclear [11,12].

Figure 1: A map of Kyrgyzstan and its bordering countries in central Asia. The dashed lines show some common drug trafficking routes.
Shortly after the HIV-1 outburst in Ukraine, IDU-A moved into the Central Russia territories and soon became widely distributed in all European and Siberian regions of Russia (up to 95%) [13], as well in Kazakhstan [14], Uzbekistan [15], Moldova [16], Belarus [17] and the Baltic countries [18,19]. Additionally, in the late 1990s, the recombinant form CRF03_AB (gagA/envB) caused a sizable HIV outbreak in Kaliningrad (Western Russia enclave); its gagA segment was derived from the IDU-A variant, but the ancestry of the envB segment is unknown. CRF03_AB is now found everywhere in Russia, with a frequency of approximately 1% [20].
Finally, HIV-1 recombinant CRF02_AG was found in Uzbekistan in 1999-2000 [15] and then in Kazakhstan [21], where it caused approximately 50% of the new cases of HIV infection in 2009 [22]. The new HIV-1 recombinant formed by CRF02_AG and IDU-A (CRF63_02A1) was registered in 2012 [23]; it is now widely distributed in Siberia and is frequently found in all other Russian regions [23,24].
Thus, the epidemic in Kyrgyzstan began against a background of a variety of HIV variants in neighboring countries.
In many ways, the HIV epidemic in Kyrgyzstan is concealed because of the stigma and low level of testing and counseling. The official figure was 5115 cases of HIV infection in 2013 [25]; according to 2012 UNAIDS estimates, the number of people living with HIV in Kyrgyzstan was 8,700 (6,000 - 13,000) [26].
As in other countries of the FSU region, the epidemic is mainly driven by injecting drug use (65.4% for the entire period of the epidemic) [10]. However, the data on the prevalence of HIV among IDUs in Kyrgyzstan are restricted to the only sentinel surveillance study. It was demonstrated that HIV prevalence among IDUs increased two times compared to 2007 (from 7.7% to 14.6%, respectively) [10] and was the highest among the Central Asian countries.
Sexual partners of HIV-infected IDUs constitute a large group of newly detected HIV cases [27]. Additionally, an HIV outbreak in a children’s hospital in Osh region in 2007 was caused by hospital-acquired infection and significantly contributed to the growth in officially recorded HIV cases [28].
In addition to the growing proportion of IDUs, other tendencies can be tracked, such as the trend in the number of cases of HIV infection among women. Since 2001, the proportion of women among people living with HIV has tripled, rising from 9.5% to 30.7% [5]. Large portions of these women are most likely IDUs themselves or are the sexual partners of infected IDUs. Another characteristic is the expansion of migration since the collapse of the USSR in 1991. According to multiple medical and sociological surveys [5,10,29-31], the majority of people migrates to Russia and other bordering countries for seasonal or other short-term work and return back to Kyrgyzstan. It is reasonably believed that this migration of labor workers is linked to the HIV epidemic in Kyrgyzstan.
The HAART programs in Kyrgyzstan started in 2005, but the coverage is still low (approximately 23% in 2011) [20,26,32]. The country has experienced a shortage in laboratory equipment and diagnostic tests for viral load and immune status monitoring in antiretroviral therapy (ART) patients. As drug resistance (DR) analysis has not yet been implemented into laboratory practice, no surveillance data on DR are available. As of 2014, no studies on the prevalence of HIV subtypes had been conducted in Kyrgyzstan. In this paper, we present the results of a joint KyrgyzRussian study conducted during 2009-2010.
Blood samples and questionnaire data were collected from 57 HIVpositive patients living in the Osh and Chui regions and Bishkek and attending the Kyrgyz Republican AIDS Centre in April 2009 – September 2010. Sampling was performed in patients as they visited for regular monitoring surveys, without any preference.
Peripheral mononuclear blood cells (PBMCs) were separated, frozen and kept in a local laboratory at -70°C until transported to analytical lab using cold chain delivery service. Each participant provided informed consent following approval from the Russian governmental ethical review committee (FWA00000621). None of the patients received ART prior to the date of sample collection, and no data on the viral load were available.
The proviral DNA extracted from the sampled PBMCs was analyzed using an in-house set of primers [30]; as a result, sequences from the PR (protease, 2253-2550 nucleotides with HXB2 strain as the reference) and RT (reverse transcriptase, 2551-2922 nucleotides) genes in the pol region and gag gene (1620-2040 nucleotides) sequences were obtained.
Sequences from PR and RT individual primers were assembled using BioEdit, version 7.0.5.3 (www.mbio.ncsu.edu/bioedit/bioedit.html). The alignment of the PR-RT sequences as well as the analysis of synonymous and nonsynonymous substitutions ratio were performed using MEGA 5.0 (http://www.megasoftware.net) [33]. The phylogenetic trees were constructed using the PhyML tool (http://www.hiv.lanl.gov) and the Maximum Likelihood method of phylogeny, the bootstrap method of branch support (1000 replicates) and the GTR+Gamma nucleotide substitution model. Optimization of the phylogenetic trees was carried out using FigTree v.1.3.1 and MEGA 5.05.
Genotyping and drug resistance mutation analyses were performed using the online programs COMET HIV-1/2 & HCV v.0.2 (http://comet. retrovirology.lu/) [34] and HIVdb Program v.7.0 (http://sierra2.stanford. edu/sierra/servlet/JSierra), respectively. The analysis of recombinant HIV-1 sequences was made with the Recombinant Identification Program (RIP) (http://www.hiv.lanl.gov/content/sequence/RIP/RIP.html) [35].
The main demographic and epidemiologic characteristics of the patients studied are presented in Table 1. The possible route of infection was determined during the course of an epidemiological survey, including questions about intravenous drug use, sexual practices and other episodes that could lead to HIV infection. Most of the patients were men (77.2%), and most of them were IDUs (97.7%). The majority of women (69.2%) were likely to be infected via heterosexual contact. The average age of the patients was 33.7; the average time since HIV diagnosis was two years (range 1-8 years).
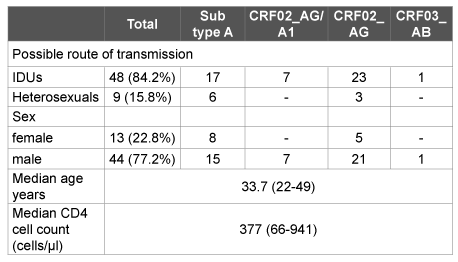
Table 1: Patient characteristics by HIV-1 subtypes
For reference, in 2010 the total number of people living with HIV in Kyrgyzstan was estimated as 4,200 [8] with most of them being men (about 75%). Injecting drug use accounted for 50–70% of cumulative HIV cases [8].The prevalence of men who have sex with men (MSM)in Kyrgyzstan is systematically underestimated due to criminalization [8,36]; in 2013 it was reported as 1.1% [36].
We evaluated preliminary data on the HIV-1 subtype distribution by the COMET tool for genotyping. According to the results, all the PRRT sequences could be divided into two groups corresponding to HIV- 1 subtype A1 and CRF02_AG, with one exception that was subtyped as CRF03_AB. For a more advanced analysis, a phylogenetic tree was constructed that included the reference strains typical for the FSU HIV infection epidemic (IDU-A, IDU-B, CRF03_AB, CRF63_02A1 [20] (Figure 2). The phylogenetic analysis of the PR-RT sequences revealed that the strains subtyped as A1 formed a large cluster with the IDU-A variants from Russia, Ukraine, Kazakhstan and Uzbekistan. Most of the CRF02_AG variants were close to each other and to the reference strains found in Uzbekistan and Kazakhstan in previous years [23,37].
Nevertheless, there were a few samples, namely, KG17, KG19, KG023, KG033, KG50, KG61, KG67, KG87, and KG99, that appeared to be outliers with regard to the two main groups of sequences. This suggested a possible recombinant structure of the genomes of these samples. To look closely into the possibility of the formation of new recombinants, an additional analysis was performed using the RIP tool. According to these examinations, two samples, KG023 and KG033, were determined to belong to the “pure” CRF02_AG group, and another seven samples were found to be formed by CRF02_AG and IDU-A variants (02_AG/A1) with each having a unique recombinant genome structure in the PR-RT region (Figure 3). The refined results of subtyping are presented in Table 1.
To better understand the structure of the recombinants, the gag genome region was analyzed using RIP in 36 samples, including all of the PR-RT 02_AG/A1 variants together with the “pure” A1 and CRF02_AG samples as controls. According to the results of the RIP analysis, the pol and gag genotypes coincided in all of the “pure” samples. For the seven 02G/A1 samples, three of them, namely KG17, KG87 and KG99, were determined to have an A1 gag region, and the remaining four samples, KG19, KG50, KG61, and KG67, were found to belong to the CRF02_AG gag genotype. These data were confirmed by phylogenetic analysis (Figure 4).
The ratio of synonymous and nonsynonymous substitutions was 0.093 in gag gene and 0.139 in pol gene.
The analysis of the drug resistance mutations in all 57 samples belonging to the naïve patients revealed four sequences (7.0%) containing mutations in the RT region, including M184I (n=1), K65R (n=1), Y181C (n=2), K103N (n=1), and G190S (n=1); no mutations in the PR region were detected.
This article presents for the first time the composition of the HIV-1 genetic variants driving the HIV epidemic in Kyrgyzstan. The authors are fully aware of the limitations of this study, which include:
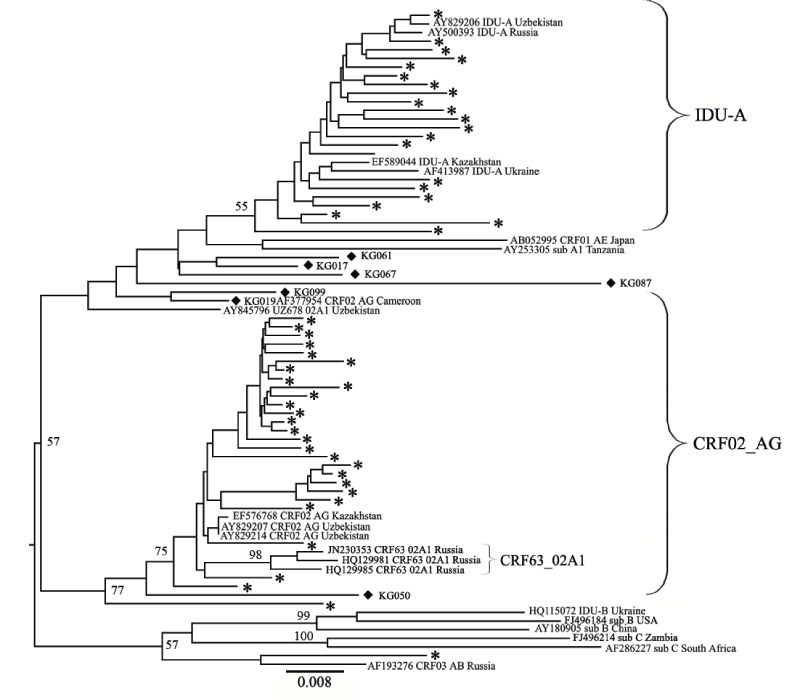
Figure 2: Phylogenetic analysis of the HIV-1 pol sequences and the joined Pro-RT region (location from start of HXB2 genome 2253-3018). The phylogenetic tree was constructed using the PhyML tool and the Maximum Likelihood method of phylogeny, the bootstrap method of branch support (1000 replicates) and the GTR + Gamma nucleotide substitution model. Optimization of the phylogenetic tree was carried out using FigTree v.1.3.1 and MEGA 5.05. Sequences obtained in this study are labeled with snowflakes. The recombinants between the CRF02_ AG and IDU-A variants (02_AG/A1) are labeled as black diamonds. Bootstrap values as percentages are indicated at the nodes. Reference samples are those named, followed by the subtype and geographical origin; two of the study samples (KG023 and KG033) are also named.
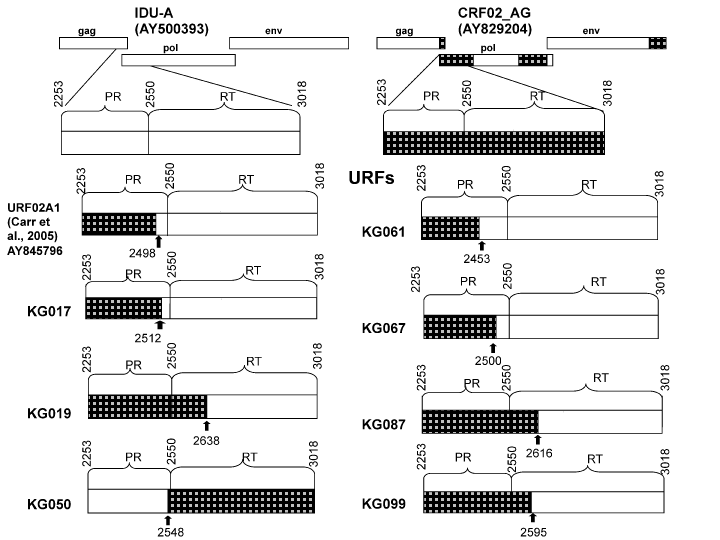
Figure 3: The structure of PR-RT genome region of the recombinants of subtype A (IDU-A) and CRF02_AG found in Kyrgyzstan. The parental strains are shown in white (IDU-A) and black (CRF02_AG). The locations of the recombinant break points (relative to HXB2) are shown as black arrows.
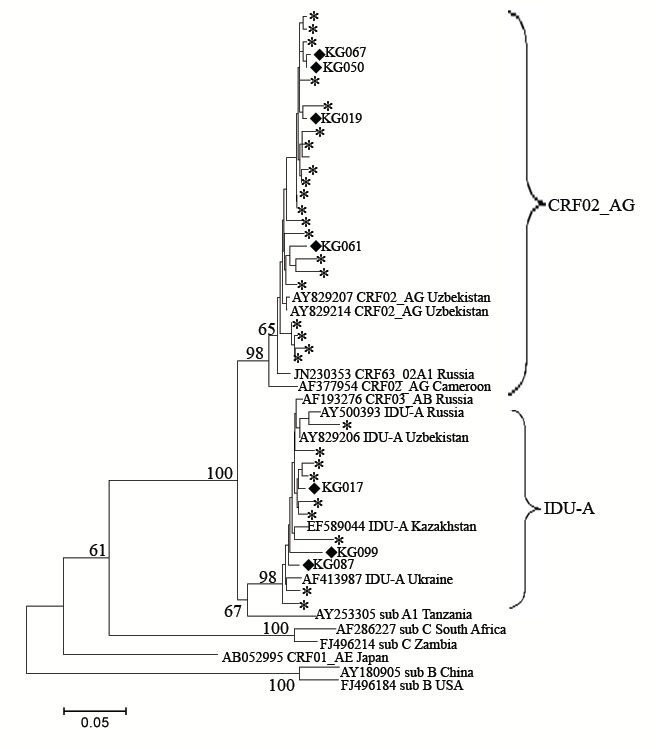
Figure 4: Phylogenetic analysis of the HIV-1 gag sequences (location from start of HXB2 genome 1624-2040). Phylogenetic tree was constructed using the PhyML tool by Maximum Likelihood method of phylogeny, bootstrap method of branch support (1000 replicates) and GTR + Gamma nucleotide substitution model. Optimization of phylogenetic tree was carried out by FigTree v.1.3.1 and MEGA 5.05. Sequences obtained in this study are labeled as snow-flakes. Unique recombinant forms which were found in this study are labeled as rhombus. Bootstrap values as percentages are indicated at the nodes.
Thus, our data reflect the status of HIV variants five years ago and may not totally reflect the current situation in Kyrgyzstan. Nevertheless, the authors decided to present the results obtained because they are currently the only data available for this country.
The study identified the complex HIV-1 molecular epidemiological patterns in Kyrgyzstan, with co-circulation of multiple subtypes, including currently circulating and unique recombinant forms. Kyrgyzstan may be the only one of the FSU countries where CRF02_AG is the predominant genetic form (45.6%; 26/57). The second-most prevalent HIV-1 genetic variant is subtype A1 (IDU-A), which is typical for all of the FSU countries (40.4%; 23/57). This finding indicates an obvious link between the HIV epidemic in Kyrgyzstan and Russia and Ukraine on the one hand and Central Asian FSU countries on the other hand [20,22]. These data are very important, considering the new information concerning the existence of subtype-associated genetic markers of HIV drug resistance. In particular, the recently described predisposition of IDU-A variants to the G190S mutation [38] leads to high levels of nevirapine and efavirenz resistance, which should be considered with regard to the adjustment of national antiretroviral treatment strategies.
An exciting finding was the unexpectedly high proportion (12.3%; 7/57) of recombinant viruses formed by the major HIV-1 groups IDU-A and CRF02_AG (02_AG/A1). Unique recombinants of this type were first discovered in Uzbekistan in 2005 [37], allowing the authors to suggest the overlapping of two drug networks, one in which CRF02_AG is prevalent and another with IDU-A. It is worth noting that the rapid growth of the epidemic among drug users in Kyrgyzstan began when these last two HIV-1 genetic variants were already widespread in neighboring countries. According to Red Crescent Society of Kyrgyzstan data [28], from 2002– 2008, the number of officially registered people with HIV in Kyrgyzstan increased 23-fold. The simultaneous introduction of both major HIV-1 variants into the population of heavy drug users could create conditions for the formation of multiple unique recombinants, which is likely why we registered at least seven potential unique recombinants forms in this relatively small study. The findings made show that the structure of the possibly unique recombinant forms detected can be quite complex, with multiple points of recombination. In this study, the genotype assignment and the recombinant analysis were performed on the basis of partial genomic sequences; thereby the recombination in other non-sequenced region cannot be excluded. To clarify this issue, new studies will be required using a complete genome analysis.
Taking into account that the HAART programs were just starting at the time of sample collection in Kyrgyzstan, another unforeseen finding was the relatively high prevalence of drug resistance mutations among naïve patients.
The statistical limitations of this study make it necessary to treat these results with caution; nevertheless, in our opinion, our results point once again to the need for systematic monitoring of drug resistance phenomenon in the region. The current study should be continued with larger numbers of samples in the future and extrapolated to the C2-V3 env genome region to increase the informational content of the data for predicting the biological and immunological properties of the viruses circulating in the region. Together with studies of the complete genome of the virus, our data will provide new knowledge concerning the unusual molecular epidemiology of HIV infection in Kyrgyzstan.
The GenBank accession numbers for the sequences described in this article are KF543275-327, KF561139-79, KF874548-71, and KF561180- 227.
The authors declare no conflict of interest.
We strongly appreciate the enthusiasm and expertise of our colleagues from the Kyrgyz Republican AIDS Centre, Bishkek, in providing epidemiological data and blood sample collection.
The research leading to these results has received funding from the International science and technology center (ISTC), project 3826 and the Russian Science foundation, project 15-15-00050. The study was supported in part by European Community's Seventh Framework Programme (FP7/2007-2013) under the project "Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)" - grant agreement N 223131.
Download Provisional PDF Here
Article Type: Research Article
Citation: Laga V, Lapovok I, Kazennova E, Ismailova A, Beisheeva N, et al. (2015) The Genetic Variability of HIV-1 in Kyrgyzstan: The Spread of CRF02_AG and Subtype A1 Recombinants. J HIV AIDS 1(2): http://dx.doi.org/10.16966/2380-5536.106
Copyright: © 2015 Laga V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access