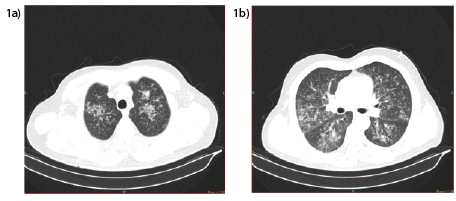
Figure1a and 1b: Thorax CT images on diagnosis (post-exposure 2nd day) showing bilateral opacities and consolidations mostly localized in the upper lobes


Sehnaz Olgun1* Emel Eryuksel1 Baran Balcan2 Buket Bayram3 Berrin Ceyhan1
1Marmara University, Medical School, Department of Pulmonology and Intensive Care, Turkey*Corresponding author: Sehnaz Olgun, Marmara University, Medical School, Department of Pulmonology and Intensive Care, Pendik Egitimve Arastirma Hastanesi, Kaynarca, 34100, Turkey, Tel: +90 505 269 31 41; E-mail: drsehnazolgun@yahoo.com
Introduction: Nitric acid also known as aqua fortis is a highly strong corrosive mineral acid. Accidental exposure to nitric acid inhalation can be fatal. In this case report, we will share our experience on successful management of a patient developing respiratory insufficiency subsequent to nitric acid inhalation.
Case: A 46 years old non smoker male patient was admitted to the emergency department (ED) with the complaint of difficulty in breathing and cough. Two days prior to being admitted to the ED an accident occurring in his workplace caused dispersion of nitric acid in the closed working environment for approximately 10 minutes. Afterwards mild symptoms of dyspnea and coughing started that progressed causing him to apply to the hospital. Upon admittance to the ED he had severe stridor and signs of bronchospasm with a SpO2 level of 85% and respiratory rate of 38/min. Immediate treatment was initiated with intra venous Methyl-prednisolone 120 mg, N-acetyl cysteine (NAC) 1200 mg enteral and bronchodilators. Noninvasive mechanical ventilation (NIV) was initiated. Within four hours the respiratory rate normalized and he hospitalized at ward for one week. End of the treatment he did not require any further oxygen supportive treatment.
Conclusion: Nitric acid exposure may be life-threatening and fatal, early administration (initial 24 hours) to hospital is essential. N-acetyl cysteine, corticosteroids, and bronchodilators may be helpful as medical treatment. Noninvasive mechanical ventilation can be use as a supportive therapy for the respiratory symptoms.
Nitric acid also known as aqua fortis is a highly strong corrosive mineral acid. The pure compound is colorless and old forms tend to acquire yellow decomposition into oxides and nitrogen and water. Commercially available nitric acid usually has a concentration between 52-68% [1]. Nitric acid reacts with most metals but the effects depend on the concentration of the acid and the nature of the metal. Diluted nitric acid behaves as a typical acid, it liberate H2 molecules after its reaction with most metals magnesium, manganese and zinc liberate H2 molecules. Other forms of nitric acid give the nitrogen oxides [2]. NO2 is also known as a reactive free radical, liberated from nitric acid during the contact of nitric acid with organic materials nitrogen dioxide Nitric oxide NO is the other oxide molecule which is a product of biological metabolism of nitric acid [3]. Nitric acid has been widely used in various industries as a solvent, pickling agent, metal refining or cleanser [4]. NO and NO2 can be created by fossil fuel combustion, volcanoes and fires. NO2 and nitric acid can cause a severe and extensive damage of mucous membranes in the body [4]. After exposure symptoms may present acute, subacute and sometimes depend on the amount of the exposed material. Clinical findings may vary from mild irritation of the upper airways to third degree burns of the respiratory tract again depend on the intensity and duration of the exposure [5]. Acute non-cardiogenic pulmonary edema, pulmonary hemorrhages are the most fatal complications of the nitric acid inhalation [6].
There are a limited number of cases in the literature with accidental exposure to nitric acid inhalation most cases being fatal [4,7]. In this case report, we will share a patient developing respiratory insufficiency subsequent to nitric acid inhalation treated successfully with noninvasive mechanical ventilation, corticosteroids and N-acetyl cysteine.
A 46 years old nonsmoker male patient was admitted to the emergency department with the complaint of difficulty in breathing and cough. Two days prior to hospital admission, he reported to be cleaning metal work instruments with nitric acid when an accident occurred causing the sudden dispersion of the nitric acid in the work environment. During the event he was alone in a closed room without any air conditioning and he couldn’t leave the room until help from outside arrived. Duration of exposure was approximately 10 minutes and afterwards his dry cough and mild dyspnea started.Two days later he sought for help from the health assistance service provided by phone line while he was at home due to worsening of his symptoms with progression to severe dyspnea and cyanosis. On arrival to the emergency department the following findings were noted: SpO2 : 85%, pulse: 120/min, blood pressure: 120/70 mmHg, respiratory rate: 38/min, severe bronchospasm and stridor. On his imaging studies carried out with chest x ray and computed tomography he had bilateral opacities and consolidations mostly localized in the upper lobes (Figure1a and 1b). He was immediately given high flow oxygen with nasal mask and rebreathing bag. His arterial blood gases values were; pH: 7.46, pO2 : 55 mmHg, pCO2 : 30 mmHg, HCO3 :28 mEq/L and SO2 : 86% at room air. Other routine blood analyses and blood counts were normal.

Figure1a and 1b: Thorax CT images on diagnosis (post-exposure 2nd day) showing bilateral opacities and consolidations mostly localized in the upper lobes
Subsequent to the findings identified the patient was administered with intravenous Methyl-prednisolone 120 mg, N-acetyl cysteine (NAC) 1200 mg enteral and bronchodilators. Noninvasive mechanical ventilation (NIV) was initiated help to decrease the working of breath. After four hours the respiratory rate was 25/min with 5 Lt oxygen with nasal mask. He was transferred to the respiratory ward and continued to the methyl prednisolone, N-acetyl cysteine and bronchodilator therapy for 7 days. At the end of the first week the patient did not require any further oxygen supportive treatment and there was no difficulty in breathing with normal lung sounds on auscultation. With complete clinical remission the patient was discharged with continued treatment of NAC 1200 mg and prednisolone. Fifteen days after discharge he was seen at the outpatient clinic and he had no symptoms and no abnormal physical examination findings. A control thorax CT on this visit showed a limited amount of consolidations and opacities (Figures 2a and 2b).
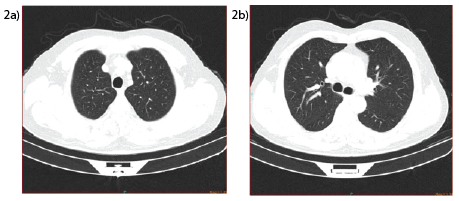
Figure 2a and 2b: control thorax CT images from the 2nd week after discharge from hospital showing resolution compared to initial images with limited amount of consolidations and opacities
Exposure to nitric acid and nitrogen dioxide may result in a wide variety of symptoms related with the duration of exposure and concentration of the solution and gases. Although nitric acid vapor, usually colorless, does not cause any protective cough reflex during the first moment of exposure while it can cause development of mucous membrane injury due to its a strongly corrosive chemical structure. Lung injury is mostly related with the oxides of the HNO3 which are known as reactive free radicals [8]. Acute inhalation injury is unlikely with commercial products, which is concentered less than 60%. Lower concentrated products are responsible for chronic exposure related injuries. Clinical presentation of patients has been grouped into three categories; i) immediate death, seen after exposure to the high concentration of acid, ii) delayed effects usually after 48 hours and iii) mild effects usually reverse shortly [9].
Nitrogen dioxide causes a structural injury mainly to the distal part of the airway, usually being at distal bronchioles and alveoli [10]. It is thought to be related with NO2 being more soluble at distal airways rather than the proximal sites [11]. There are several different mechanisms for NO2 toxicity. Through triggering the lipid peroxidation and oxidation it causes formation of liberated free radicals. Additionally, it also leads to increased membrane permeability and inhibits cellular metabolism resulting in damage of intracellular organelles [3]. These effects result in cellular damage occurring in the alveolar area after inhalation. A cumulative excessive exposure effects, mostly type I cells and this causes an impaired gas exchange, but repeated or prolonged exposure usually affects type II cells more and resulting in interstitial thickening [3]. The microscopic findings of the damage are related with increased vascular permeability and edema [12]. Alveolar capillaries contain necrotic endothelial cells and degranulated neutrophils. The release of neutrophil enzymes and free radicals increase the progression of injury [12]. Due to increased permeability of the capillaries edema fluid is rich in albumin, IgG and IgM [7].
Dyspnea, wheezing, cough, palpitation and chest pain are the common symptoms seen in these patients. Additionally, nausea, diaphoresis and vomiting may also be seen and severity of symptoms is related with duration of exposure. Symptoms can occur within a few minutes to hours and can persist up to weeks. Delayed symptoms can start up to 12 hours after exposure and usually are related with laryngospasm and bronchospasm. Clinical presentations can include severe cases such as; chemical pneumonitis, severe pulmonary edema and respiratory failure [4]. Similar to previous reported cases, our patient’s symptoms were also characterized by respiratory discomfort, cough and dyspnea and he was admitted to the hospital 2 days later.
All published case reports about nitric acid and nitrogen dioxide exposure are enunciated as occupational accidents. Similarly, our patient had exposure while cleaning his working place. First case series for nitric acid inhalation reported death in all 3 cases of pulp-mill workers due to accidental nitric acid explosion [7]. All three workers inhaled heavily dust and fumes after a nitric acid tank explosion. They had no significant symptoms or physical examination findings shortly after the explosion, but within 4 to 6 hours they were rushed to the hospital with respiratory failure secondary to pulmonary edema. They had frankly fluid escaping from the mouth and nose, were rapidly intubated and instituted mechanical ventilation, but all died less than 24 hours [7]. Necropsy findings showed that the lungs were firm and up to five times heavier than normal, alveolar space was filled with protein rich fluid, capillary enlargement, interstitial edema and hyaline membranes also were detected [7]. Murphy et al. [4] reported a case after tank cleaning with a solution of 50-70% concentrated nitric acid. Although he did not report on complain about any nasal or orofaringeal irritation during the cleaning process the patient was admitted to the emergency department 5 hours after exposure. The patient developed severe hypoxemia at the 10th hour and transferred to the ICU for mechanical ventilation support. Despite the therapies the patient died on the 3rd day of the hospital admission. Autopsy findings showed an edematous lung with enlarged vessels and intra-alveolar proteinaceous debris. Jayalakhmi et al. and Murphy et al. [4,9] also reported different clinical presentations of nitric acid exposure case reports Murphy et al. [4] reviewed the literature about nitric acid exposure and found that immediate exposure is rare and fatal, usually symptoms occur shortly after exposure despite no presentation with mucosal or ophthalmic irritation and that symptoms were mostly related to airway irritation. This was similar to our patient who reported no upper airway or ophthalmic irritation. Shin et al. [5] reported a patient recovered with extracorporeal life support because of fatal edema after exposure to nitric acid. Recently Yoon-Sook et al. [13] reported an unexpected hypotension and tachycardia during propofol infusion for extracorporeal membrane oxygenation cannulation of a patient with nitric acid exposure. They claimed that propofol may stimulate nitric oxide activity and shows a concentration-dependent increase in NO production by neutrophils and causes a severe hypotension. Thus, anesthesiologist must be aware of clinical features of nitric acid inhalation injury.
Methyl prednisolone NAC and bronchodilators may be effective for mild cases. However, severe cases may need additional invasive mechanical ventilation or ECLS, most rapidly progressive and fatal. Fortunately our patient did not need invasive mechanical ventilation or extracorporeal membrane oxygenation and gave good response to corticosteroids, NAC and NIV.
After reviewing the literature and experience gained from the management of this patient, it seems that NAC, corticosteroids, and bronchodilators are helpful in the case we described. Supportive treatment in the form of oxygen inhalation with noninvasive ventilation was required in our case. Consideration of early hospitalization of patients is imperative since the symptoms may be lacking. It would be helpful to keep asymptomatic patients under observation for at least 24 hours as during the initial phase symptoms may progress requiring treatment. Though, the role of corticosteroid is dubious the mechanism by which it reduces inflammation might have some beneficial effect in inhalation poisoning; therefore, we used corticosteroids and we have seen it to be helpful.
Download Provisional PDF Here
Article Type: Case Report
Citation: Sehnaz O, Eryuksel E, Balcan B, Bayram B, Ceyhan B (2016) Accidental Nitric acid Gas Exposure and Respiratory Effect. J Epidemiol Public Health Rev 1(6): doi http://dx.doi. org/10.16966/2471-8211.130
Copyright: © 2016 Sehnaz O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access