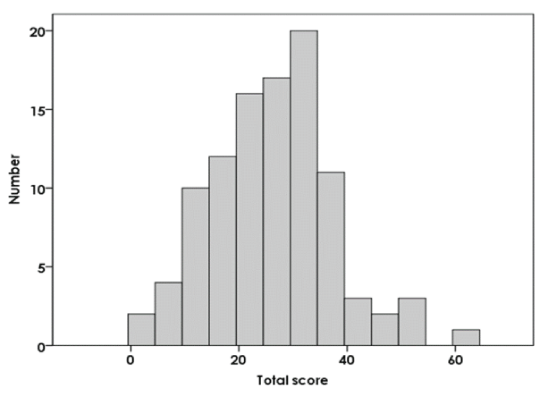
Figure 1: Frequency distribution of fin-Q total score in patient group. The final questionnaire’s 16 items are each evaluated on a five-grade scale (range, 0–64). The patient group’s score ranges from a minimum score of 2 to a maximum score of 60.


Rena Nakayama Akira Nishiyama* Masahiko Shimada
Orofacial Pain Management, Oral Health Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Japan*Corresponding author: Akira Nishiyama, 1-5-45, Yushima, Bunkyo-ku, Tokyo 113-8549, Japan, Tel: +81-3-5803-5713; Fax: +81-3-5803-5713; E-mail: anishi.tmj@tmd.ac.jp
Objective: To create a new quality of life (QOL) questionnaire specifically for temporomandibular disorder (TMD) patients.
Materials and Methods: From April 2016 to March 2017, individuals undergoing initial examinations or treatment for TMD at our dental hospital (i.e., patient group) and individuals with no diagnosis or subjective symptoms of TMD (i.e., control group) completed self-assessed questionnaires (ethical approval no. 1285). We compared intergroup differences in the mean scores, and ranked questions by the size of the difference. We then created a novel 16-item questionnaire. Cronbach’s alpha was 0.950. Correlation of the total scores of the questionnaire with the numerical rating scale (NRS; to assess pain) and with the Hospital Anxiety and Depression Scale (HADS; to assess TMD-related psychosocial and functional disturbances) was evaluated using correlation coefficients.
Results: Participants were ≥ 20 years old. The patient group and control group comprised 103 participants and 173 participants, respectively. In both groups, the correlation coefficients for the questionnaire’s total scores with the NRS (0.6-0.8) and with the HADS showed moderate or higher correlations.
Conclusions: The questionnaire strongly correlated with the patients’ pain intensity and subjective level of disturbance and may be used as an index for TMD patients’ QOL level or therapeutic effect.
Diagnostic criteria for temporomandibular disorders; Numerical rating scale; Oral health-related quality of life; Pain intensity; Selfassessed questionnaire
Temporomandibular disorders (TMD) are a subclass of musculoskeletal disorders resulting from dysfunctions of the stomatognathic system that affects the masticatory muscles, temporomandibular joint, and orofacial structures [1]. Temporomandibular disorders are multifactorial and have a positive natural course and an incidence rate of approximately 5–12% [2,3]. Symptoms include pain, joint sound, and limited mouth opening, while treatments include behavioral modification, splint and pharmaceutical therapies. The goal of TMD therapy is focused on improving the symptoms rather than providing a complete cure. Therefore, the extent to which TMD symptoms affect the daily life of TMD patients’ needs to be assessed.
Most studies have been focused on evaluating the degree of mental and physical disturbances. The visual analog scale (VAS) or the numerical rating scale (NRS) is often used to assess pain. Moreover, the extent of mouth opening is used as an index that can express the disturbance caused by TMD. However, pain intensity and the extent of mouth opening do not necessarily reflect the deteriorating level of a patient’s quality of life (QOL).
Assessing patients’ QOL has recently become an important part of medical care. In TMD, a patient’s own assessment has great relative importance for whether symptoms have improved. Therefore, much research has been conducted on the QOL assessment of TMD patients [4,5].
Previous research has highlighted the need for high-quality evidence to create an outcome measure with validity and reproducibility that is focused on TMD patients [6-8]. Several scales for assessing QOL in patients with TMD have been created, based on questions from scales such as the Oral Health Impact Profile (OHIP) and the Research Diagnostic Criteria for TMD (RDC/TMD). A questionnaire’s responsiveness for assessing QOL in TMD patients can differ, depending on the patient’s country or culture [9].
Being able to properly evaluate problems especially for TMD patients would allow medical professionals to assess their patients’ conditions (severity, reduced QOL, etc.). Sugisaki et al. [10] created a questionnaire on TMD pain-related limitations of daily function (LDF-TMD) in Japanese patients. The questionnaire’s 10 items were each evaluated on a five-grade scale (range, 0–40 points). However, the mean score for actual TMD patients was 13.6 [11], which indicated it cannot be used to assess the QOL of TMD patients in detail. In this situation, multiple questionnaires would need to be combined to qualitatively assess the QOL of TMD patients. However, more items for question would increase the burden on patients [12,13]. For this reason, we developed a QOL questionnaire specifically for TMD patients so that only one evaluation needs to be performed.
A survey using a self-assessed questionnaire was administered to individuals from April 2016 to March 2017. The study participants consisted of 103 patients in the patient group and 173 individuals in the control group. All participants were 20 years old or older. The patient group comprised patients who came for initial examinations or were being treated at the Temporomandibular Joint Clinic at the Tokyo Medical and Dental University Dental Hospital (Tokyo, Japan). Patients were selected who had received a definitive diagnosis of TMD, based on the diagnostic criteria for TMD (DC/TMD) [14], and whose pain had persisted for at least 3 months. The control group comprised employees, students, and graduates of the university who had never been diagnosed with TMD and had none of its subjective symptoms. Patients were excluded if they met any of the following criteria: (1) they had a systemic disease such as rheumatism, (2) they had acute symptoms (i.e., acute inflammation of the orofacial area), or (3) they regularly used antidepressants, antianxiety agents, or psychotropic agents. After patients underwent outpatient examinations or care, an investigator explained the survey and obtained in writing their consent to participate in the study. A questionnaire was then distributed and collected after the patient completed it. For the control group, an investigator followed the same procedures as with the patient group regarding explanations and other matters. The questionnaires were administered in areas such as classrooms and laboratories, and were collected after participants completed them. No personal information was collected that could identify an individual (e.g., name, address, patient number). This study was approved by the ethical screening committee of the Faculty of Dentistry at Tokyo Medical and Dental University (approval no. 1285).
To create a temporary questionnaire (temp-Q), questions that we judged were highly relevant to TMD patients’ QOL were selected from the OHIP, Limitations of Daily Function in TMD Questionnaire (LDFTMDQ), DC/TMD, and other questionnaires made specifically for TMD patients [4-11,13-16] (Table 1). The temp-Q followed the format of the OHIP and other existing questionnaires by using a five-point Likert scale (0=‘not at all’; 1=‘almost never’; 2=‘sometimes’; 3=‘often’; 4=‘always’). Pain intensity and limitations on daily activity due to pain were evaluated using the 11-point NRS which ranges 0–10 points. Pain intensity was assessed as the maximum pain and average pain felt over the past 1 month with 0 indicating ‘no pain’ and 10 indicating ‘the strongest pain imaginable.’ Limitations on daily activity due to pain were divided into limitations on daily life overall, on work, and on diet with 0 indicating ‘no problems’ and 10 ‘not able to do anything.’ Moreover, depression and anxiety were assessed using the HADS.
| No | Item |
| Q1 | Have you had difficulty talking for a long time, including talking on phone? |
| Q2 | Have you had difficulty opening your mouth when eating big pieces of foods such as a hamburger or sushi? |
| Q3 | Have you had difficulty grinding thin foods such as seaweed or lettuce? |
| Q4 | Have you had difficulty clenching teeth when participating in sports? |
| Q5 | Have you had difficulty brushing your back teeth? |
| Q6 | Have you had difficulty yawning? |
| Q7 | Have you experienced orofacial jaw muscle fatigue or pain when you are awake? |
| Q8 | Have you had difficulty in performing your daily activity at home, work or school? |
| Q9 | Have you had difficulty falling asleep at night? |
| Q10 | Have you had difficulty sleeping through the night without waking up? |
| Q11 | Have you had to interrupt meals? |
| Q12 | Have you had difficulty chewing any foods? |
| Q13 | Have you had to avoid eating some kinds of foods? |
| Q14 | Have you had headaches? |
| Q15 | Have you felt anxious and troubled? |
| Q16 | Have you felt tense? |
| Q17 | Have you found it difficult to relax? |
| Q18 | Have you been upset? |
| Q19 | Have you been self-conscious? |
| Q20 | Has your concentration been affected? |
| Q21 | Has your jaw pain made you feel miserable? |
| Q22 | Have you felt depressed? |
| Q23 | Have you had sore spots in your mouth? |
| Q24 | Have you had sensitive teeth (for example, because of hot or cold foods or drinks)? |
| Q25 | Have you had problems with your bite? |
| Q26 | Have you had pain during talking? |
| Q27 | Have you felt that your dentures or crowns have not been fitting properly? |
| Q28 | Have you allowed your upper and lower teeth to make continuous contact during work or when focusing on one thing? |
| Q29 | Have you experienced other people pointing out that you make teeth-grinding sounds during sleep? |
| Q30 | Have you worried or fretted at work, school or home? |
| Q31 | Have you felt tense in a personal relationship at your work, school, or home? |
| Q32 | Have you had stress from work, school, home, or a personal relationship? |
| Q33 | Have you felt anxious about work, school, home, or a personal relationship? |
| Q34 | Have you felt fatigued, even after sleeping or taking a rest? |
Table 1: Temporary questionnaire (temp-Q). The participants answered the question items on a five-point numeric rating scale: 0=‘not at all’; 1=‘almost never’; 2=‘sometimes’; 3=‘often’; 4=‘always.’
For the patient group, items concerning the pathological diagnosis of TMD and pain duration were added. To exclude people at high risk of TMD from the control group, we used a screening questionnaire for TMD (SQ-TMD) [17].
We entered the obtained data into SPSS version 21.0 (IBM Japan, Tokyo, Japan) to create a database for statistical analysis. Scores for each temp-Q item were compared between the patient and control groups using the Mann–Whitney U test. Items that did not show statistically significant differences were eliminated. The mean scores for each question were calculated; differences between the patient and control groups’ mean values were determined; and the questions were ranked, based on the size of the difference. Items with differences less than 1.0 were eliminated. A final questionnaire (fin-Q) was created using the remaining items.
Factor analysis of the fin-Q was conducted (such as principal factor analysis, promax rotation) to investigate the constructs. To examine internal consistency, Cronbach’s alpha was calculated overall and for each construct (i.e., reliability analysis). Furthermore, the fin-Q total scores were calculated, and correlation coefficients between the score distribution and NRS values and HADS scores were determined. Sex differences in the fin-Q total scores were examined and correlation coefficients between the fin-Q total score and age were determined. SPSS version 21.0 (IBM Japan) was used for the statistical analysis and a value of p<0.05 indicated a statistically significant difference.
Members of the patient and control groups who had incomplete data were excluded. Based on the SQ-TMD results, members of the control group who were assessed as having a high risk of TMD were also excluded. As a result, there were 101 people in the patient group (mean age, 50.7 ± 14.9 years; sex, 83 females and 18 males) and 131 people in the control group (mean age, 40.1 ± 13.2 years; sex, 59 females and 72 males). Based on the TMD pathological classifications in the patient group, 85 patients had arthralgia; 63 patients, myalgia; 36 patients, disk displacement with reduction; and 40 patients, disk displacement without reduction. The HADS scores were not significantly different between the groups (Table 2).
| Total | Patient | Control | p | ||
| N=232 | N=101 | N=131 | |||
| Anxiety | Normal | 165 (71.1) | 70 (69.3) | 95 (72.5) | 0.538 |
| Borderline abnormal | 39 (16.8) | 20 (19.8) | 19 (14.5) | ||
| Abnormal | 28 (12.1) | 11 (10.9) | 17 (13.0) | ||
| Depression | Normal | 179 (77.2) | 79 (78.2) | 100 (76.3) | 0.939 |
| Borderline abnormal | 43 (18.5) | 18 (17.8) | 25 (19.1) | ||
| Abnormal | 10 (4.3) | 4 (4.0) | 6 (4.6) |
Table 2: Hospital Anxiety and Depression Scale. The data are presented as the number (percentage)
Table 3 shows the ranking of questions in the patient and control groups. The ranking was based on differences in the mean scores (i.e., the patient group minus the control group). Questions 30–34 were excluded from the ranking table because these scores were not significantly different between the groups. Items for which the difference in the mean scores was less than 1.0 were also eliminated. Thus, 16 items were used to create the fin-Q (Table 3).
|
Mean | P minus C |
|
|
Patient (P) |
Control(C) |
|
| Q6* | 2.26 | 0.10 | 2.16 |
Q12* |
2.21 | 0.13 | 2.08 |
| Q7* | 2.01 | 0.29 | 1.72 |
Q13* |
1.84 | 0.13 | 1.71 |
Q15* |
1.91 | 0.21 | 1.70 |
Q16* |
1.75 | 0.23 | 1.52 |
Q17* |
1.67 | 0.23 | 1.44 |
Q14* |
1.59 | 0.21 | 1.38 |
Q25* |
1.95 | 0.61 | 1.34 |
Q22* |
1.45 | 0.13 | 1.32 |
Q23* |
1.56 | 0.36 | 1.20 |
| Q9* | 1.31 | 0.19 | 1.12 |
Q10* |
1.45 | 0.33 | 1.12 |
| Q3* | 1.16 | 0.08 | 1.08 |
Q20* |
1.28 | 0.22 | 1.06 |
Q11* |
1.12 | 0.08 | 1.04 |
| Q5 | 1.08 | 0.13 | 0.95 |
| Q21 | 1.07 | 0.12 | 0.95 |
| Q4 | 1.03 | 0.11 | 0.92 |
| Q18 | 1.03 | 0.12 | 0.91 |
| Q26 | 1.00 | 0.10 | 0.90 |
| Q27 | 1.09 | 0.22 | 0.87 |
| Q19 | 1.01 | 0.15 | 0.86 |
| Q8 | 0.94 | 0.12 | 0.82 |
| Q1 | 0.77 | 0.06 | 0.71 |
| Q28 | 2.30 | 1.62 | 0.68 |
| Q29 | 1.36 | 0.79 | 0.57 |
| Q32 | 1.97 | 1.53 | 0.44 |
| Q33 | 1.78 | 1.36 | 0.42 |
| Q24 | 1.42 | 1.03 | 0.39 |
| Q34 | 2.15 | 1.81 | 0.34 |
| Q31 | 1.86 | 1.55 | 0.31 |
| Q30 | 1.98 | 1.69 | 0.29 |
| Q2 | 2.39 | 2.17 | 0.22 |
Table 3: Item ranking
*items selected for final questionnaire (fin-Q)
The fin-Q total score of the patient group was 26.5 ± 11.1 (minimum, 2; maximum, 60) and that of the control group was 3.5 ± 5.2 (minimum, 0; maximum, 25). Factor analysis divided the items into 2 complexes (Table 4). The factor loading of Question 7 was virtually the same in both complexes. When Question 7 was included in Factor 1, Cronbach’s alpha was 0.947 for Factor 1 and 0.907 for Factor 2. When Question 7 was included in Factor 2, Cronbach’s alpha was 0.946 for Factor 1 and 0.917 for Factor 2. When Question 7 was included in Factor 2, Cronbach’s alpha of Factor 1remained virtually unchanged, but Cronbach’s alpha for Factor 2 increased; therefore, we decided to include it in Factor 2. Based on this result, we defined Factor 1 as ‘psychosocial disturbance’ and Factor 2 as ‘functional disturbance.’ Cronbach’s alpha for all 16 items was 0.950.
| Item | Factor 1 | Factor 2 | |
| Psychosocial disturbance | Q16 | 0.979 | –0.063 |
| Q17 | 0.893 | 0.043 | |
| Q15 | 0.888 | 0.036 | |
| Q20 | 0.799 | 0.047 | |
| Q9 | 0.739 | 0.016 | |
| Q22 | 0.705 | 0.152 | |
| Q10 | 0.663 | 0.032 | |
| Q14 | 0.567 | 0.254 | |
| Functional disturbance | Q7 | 0.413 | 0.408 |
| Q13 | –0.053 | 0.911 | |
| Q12 | 0.065 | 0.866 | |
| Q11 | 0.017 | 0.773 | |
| Q6 | 0.039 | 0.736 | |
| Q3 | 0.048 | 0.717 | |
| Q23 | 0.297 | 0.477 | |
| Q25 | 0.289 | 0.386 |
Table 4: Factor analysis results
Figure 1 shows the frequency distribution of the fin-Q total scores in the patient group. The Kolmogorov–Smirnov normality test obtained p=0.200, which indicated anormal distribution.

Figure 1: Frequency distribution of fin-Q total score in patient group. The final questionnaire’s 16 items are each evaluated on a five-grade scale (range, 0–64). The patient group’s score ranges from a minimum score of 2 to a maximum score of 60.
The correlation coefficients for fin-Q total scores with the NRS values and with the HADS scores in the patient and control groups showed moderate or higher correlations in all instances (Table 5). A significant sex difference in the fin-Q total scores was observed (Table 6). The correlation coefficients of patients group and control group between fin-Q total score and age were 0.171 (P=0.088) and 0.166 (P=0.097), respectively.
| Correlation coefficient (P) | |||||||
| HADS | Pain intensity | Limitation by pain | |||||
| Anxiety | Depression | Maximum | Average | Daily activities | Work | Chewing | |
| Total | 0.253 (<0.001) | 0.133 (0.042) | 0.792 (<0.001) | 0.798 (<0.001) | 0.766 (<0.001) | 0.679 (<0.001) | 0.810 (<0.001) |
| Psychosocial disturbance | 0.299 (0.003) | 0.183 (0.005) | 0.729 (<0.001) | 0.744 (<0.001) | 0.713 (<0.001) | 0.645 (<0.001) | 0.736 (<0.001) |
| Functional disturbance | 0.193 (0.003) | 0.094 (0.154) | 0.806 (<0.001) | 0.808 (<0.001) | 0.768 (<0.001) | 0.664 (<0.001) | 0.839 (<0.001) |
Table 5: Correlation between the fin-Q score and the HADS and NRS score
| Sex | N | Mean (SD) | P | |
| Patient | Men |
18 | 17.7 (8.7) | <0.001 |
| Women | 83 | 28.4 (10.7) | ||
| Control | Men | 72 | 3.1 (5.2) | 0.299 |
| Women | 59 | 4.1 (5.3) |
Table 6: Comparison of the fin-Q total score based on sex
The objective of this study was to develop a new QOL questionnaire specifically for TMD patients. Compared to existing questionnaires, our questionnaire showed a high level of correlation with the patients’ subjective level of daily life, which indicated that we were successful in creating a valid candidate questionnaire.
Numerous questionnaires have been created to evaluate QOL. The Medical Outcomes Study (MOS) 36-item Short-Form Health Survey (SF36) is not specific to any disease, and can be used to evaluate QOL in healthy people [18-20]. The General/Geriatric Oral Health Assessment Index (GOHAI) is an oral health-related questionnaire that was originally created for elderly people [21]; however, its reliability and validity in other age groups has been demonstrated in several countries [22-25].
The most commonly used oral health-related QOL questionnaire is the OHIP. It has been modified to apply to implant patients, edentulous patients, and patients with partial dentures. The OHIP has a large score range; therefore, it can reflect small changes. Using the OHIP for TMD patients has been researched, but it does not sufficiently assess changes in TMD patients’ symptoms. Moufi et al. [15] found that within the OHIP total score range (0–196), the mean score of TMD patients was 60.6 (standard deviation [SD] =31.6). Rener-Sita et al. [26] found that the mean score of TMD patients was 44.0 (SD=37.5). Kothari et al. reported that the mean score of TMD patients was 80.8 (SD=44.3) [27]. These findings indicate that the OHIP contains many items that are unnecessary when applied to TMD patients, and few items are related to TMD.
The investigators in one study [15] ranked the OHIP items to select items with strong connections to patients with TMD, based on the strength of their relationship to TMD, and clarified which items were strongly related in patients with TMD. To create a questionnaire specifically for TMD patients, items would have to be added and eliminated from the OHIP. However, in the literature, we were unable to find any studies, including the Moufti study [15] that has used the OHIP questionnaire to create a new questionnaire.
Although the LDF-TMDQ, a questionnaire designed specifically for Japanese TMD patients with pain, was previously reported [11], its low mean score of 13.6 (SD=5.8) and little variation would make it difficult to use to appropriately evaluate the QOL of individual TMD patients or to assess in detail the amount of improvement from therapy. The LDFTMDQ does not contain any psychological items; therefore, it needs to be combined with the HADS or other scales to create a multidimensional questionnaire, which would make assessment more difficult. The aforementioned findings indicate that the existing QOL indices are insufficient for several reasons. Therefore, we created a new QOL questionnaire specifically for TMD patients.
In the present study, the selected items demonstrated stronger correlations to the QOL of TMD patients, compared to items used in previous studies. The questionnaire was evaluated via the breadth of the score distribution and correlation coefficients with the NRS.
The maximum fin-Q score was 64. The study participants’ responses in the present study showed a wide distribution and ranged from 2–60. The score distribution was wider than that of the OHIP and another QOL index for TMD patients. Individual patients exhibited varying levels of disturbance. The fact that this questionnaire could be applied to patients with small to large disturbance levels over a wide distribution indicated that it was easily able to reflect these differences. Therefore, it may also be able to reflect differences in the deterioration levels of a patient’s QOL from TMD symptoms.
The correlation coefficients between the fin-Q scores and NRS were 0.6–0.8, which showed a moderate or greater level of correlation. Sugisaki et al. [10] examined the extent that QOL questionnaire scores were correlated with pain intensity and subjective levels of disturbance, and found that the LDF-TMDQ exhibited correlation coefficients of 0.3– 0.6. Furthermore, the OHIP-TMD questionnaire created by Durham et al. [16] exhibited a correlation coefficient of 0.576 with VAS scores for current pain. Compared to the questionnaires used in the Sugisaki and Durham studies, the results of the present study presented a stronger correlation. This finding indicates that the fin-Q may assess the intensity of pain and degree of disturbance caused by pain more accurately than existing questionnaires.
In the present study, there were significant differences between the patient and control groups in regard to age and female sex. A significant correlation coefficient between age and the fin-Q total score was not observed in the patient group. Therefore, it appears that age had little impact.
In regard to the sex differences, a significant difference in fin-Q total scores was observed in the patient group, but not in the control group. Women generally tend to have higher levels of disturbance in oral healthrelated QOL, compared to men [28-30]. Moreover, the pain mechanism is influenced by many factors, which may account for the sex difference [31- 36]. The aforementioned findings suggest that different reference values for men and women may need to be created when using the questionnaire created in the present study.
In the future, repeat testing needs to be conducted, whether the same symptoms receive the same scores needs to be determined, and reproducibility needs to be examined. If this questionnaire demonstrates validity, it may be useful in assessing the effects of treatment. In addition, this questionnaire could be used as a base to develop a questionnaire for patients with orofacial pain.
We examined QOL questionnaire items among TMD patients and created a new QOL questionnaire comprising 16 items on areas such as difficulty in chewing food. This questionnaire correlated strongly with the patients’ pain intensity and subjective level of disturbance, which suggests it, may be possible to use it as an index for TMD patients’ QOL level or therapeutic effect.
Download Provisional PDF Here
Article Type: Research Article
Citation: Nakayama R, Nishiyama A, Shimada M (2017) Creating a Quality of Life Index for Patients with Temporomandibular Disorders. Int J Dent Oral Health 3(5): doi http://dx.doi.org/10.16966/2378-7090.241
Copyright: © 2017 Nakayama R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access