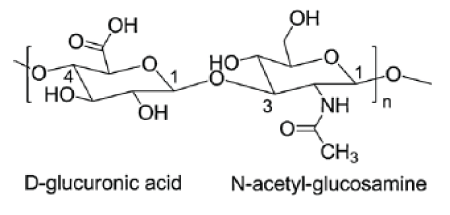
Figure 1: Structure of the disaccharide repeating unit in HA.

Mohammed Al-Sibani1* Ahmed Al-Harrasi2 Reinhard HH Neubert3
1Faculty of Science and Art, Department of Biological Science and Chemistry, University of Nizwa, Birkat Al-Mouz, Nizwa, Sultanate of Oman*Corresponding author: Mohammed Al-Sibani, Faculty of Science and Art, Department of Biological Science and Chemistry, University of Nizwa, Birkat Al-Mouz, Nizwa 616, Sultanate of Oman, E-mail: sibanimm@unizwa.edu.om
Introduction: Hyaluronic acid (HA) is a high-molecular weight polymer with many applications in cosmetic and human medicine. HA is witnessing an increasing demand over recent years and it can be found in two forms; linear and chemically cross-linked. The cross-linked HA is more durable than linear HA after administration into the human body. Characterizing the two forms of HA has become a matter of great importance.
Objective: The objective of this work is to characterize linear and cross-linked HA with data generation on their structural composition, similarities and differences using various analytical techniques.
Method: A linear hyaluronic acid solution and a cross-linked hyaluronic acid scaffold modified with 1,4-butanediol diglycidyl ether (BDDE) were prepared following a reported method with a little modification. The two formulations were characterized using FTIR, ESI-MS, NMR, and SEM.
Results: Data obtained from all techniques showed different characteristics and chemical structures. For instance, the cross-linked BDDE-HA appeared with much less peak intensity at about 3343 cm-1 in FTIR, higher mass-to-charge ratio (m/z) in ESI-MS, an additional distinctive peak at 1.5 ppm in NMR and a more porosity structure in SEM compared to the linear HA.
Conclusion: The matrix structures of linear and cross-linked HA have different characteristics. These findings were identified and confirmed via applications of four different analytical techniques.
Hyaluronic acid; Cross-linking; Chemical cross-linker; Enzymatic degradation
HA-Hyaluronic Acid; BDDE-1,4-Butanediol Diglycidyl Ether; BTH-Bovine Testicular Hyaluronidase
Hyaluronic acid (HA) also known as hyaluronan (Figure 1) is a high-molecular weight, naturally occurring biodegradable polymer. HA is a linear (un-branched) and non-sulphated glycosaminoglycans (GAG). It is composed of repeating disaccharide units of N-acetyl-Dglucosamine and D-glucuronic acid chemically linked by alternating glycosidic bonds β-(1, 4) and β-(1, 3) [1-3].

Figure 1: Structure of the disaccharide repeating unit in HA.
HA is widely distributed throughout the human body and it forms a major element in the extracellular matrix, ECM [4-6]. It is present in almost all biological fluids including synovial fluid and the vitreous humor of the eye [7]. The larger amount of HA is found in the skin and it approximately contains more than 50 % of the total content within the body [8]. In 1942, Endre Balazes was the first man who used HA in a commercial purpose as a substitute for egg white in bakery products [9]. Over the last two decades, HA has become a material of great importance in modern medicine and it has been widely employed in tissue engineering and cosmetic surgery [10,11]. However, most of these applications are not addressed with native or liner HA because the linear HA does not remain in human body for prolonged periods due to its in vivo rapid degradation and poor mechanical properties [10,12,13].
The half-life of linear HA in the skin is about 12 hours and it is rapidly broken down by the enzyme hayluronidase [14]. Therefore, linear HA should be stabilized via cross-linking methods to ensure a longer residence time within the soft tissue after administration into the body. HA cross-linking refers to a process by which HA chains are chemically bound with a chemical cross-linker through one of the HA functional groups including (-OH, -COOH, -NHCOCH3). Chemical cross-linking targeting (-OH) is commonly practiced in the industry of HA fillers because it preserves the polyanionic nature of HA [15-18]. A number of chemical cross-linkers have been addressed for HA cross-linking, however, the 1,4-butanedioldiglycidylether (BDDE) is the most common one due to the epoxide groups that preferentially react with hydroxyl groups of HA and form ether bonds [19]. The result of HA chemical cross-linking is a three-dimensional network that could retain water within its cross-linked network for a longer time but it does not dissolve in water. The cross-linked HA has a wide scope of cosmetic and medical applications in comparison to the linear HA which has few applications restricted in drugs administration and daily skin care products. For instance, the crosslinked HA has been widely employed in tissue engineering because it provides three-dimensional scaffolds which allow nutrients and cellular waste to be diffused through it [20]. In fact, the chemically cross-linked HA vs native or linear HA are more stable and show much higher resistance against enzymatic degradation. Treatment of osteoarthritis is also a major biomedical application of cross-linked HA, where the viscoelastic property of synovial fluid in the joints decreases over the time [21]. Recently, the cross-linked HA has been involved in drug delivery [22]. The HA drug carriers overcome the limitation of other polymeric carriers which are not biodegradable or do not have potential drug loading. In cosmetic industry, the crosslinked HA has also been used as an anti-aging product. The crosslinked HA fillers have become very popular in correcting facial folds and producing a younger facial appearance. They achieve a substantial tissue augmentation into the affected skin and remain swollen in tissue for longer time [23].
Currently, various commercial cross-linked HA fillers are available in the market which have been approved by the US Food and Drug Administration (FDA) [24-26]. Common examples include Restylane, Perlane from Q-Med (Uppsala, Sweden); Juvederm and Surgiderm from Corneal (Pringy, France). Special attention has been paid for proving the occurrence of chemical cross-link in HA-based products particularly for those employed in skin treatment and augmentation. In fact, differentiating linear from cross-linked HA forms a major challenge in chemical analysis due to sample complexity, structure similarity and paucity of methodologies. Generally, the most common methods used in the analysis of cross-linked glycosaminoglycans include Fourier-transformed infrared (FT-IR) and nuclear magnetic resonance spectroscopy (NMR). On other side, several analytical assays such as swelling ratio and in-vitro enzymatic degradation test can also be used to confirm polymer modification. Cross-linked polymers usually show slower degradation rate and lower swelling ratio compared to linear polymers due to the formation of bridges and intermolecular bonds between the polymer chains and the chemical cross-linker.
In a previous study, we investigated the effect of mixing approach on BDDE-HA hydrogel degradation and swelling behavior [27]. In this study, however we aimed to characterize the linear and crosslinked HA using Fourier-transformed infrared (FT-IR), electrospray ionization mass spectrometry (ESI-MS), proton nuclear magnetic resonance spectroscopy (H1NMR) and scanning electron microscopy (SEM). The cross-linker used in the reaction was 1,4-butanediol diglycidylether (BDDE) because it is the most common chemical cross-linker incorporated in HA medical and cosmetic products. The cross-linking process was carried out in strong alkaline conditions following a method described by Malson T et al., Piron E et al. [28,29].
The hyaluronic acid powder with an average molecular weight 1,000,000 Da was obtained from Vivatis Pharma (Hamburg, Germany). The chemical cross-linker 1,4-butanediol diglycidyl ether (BDDE) and the enzyme bovine testicular hyaluronidase BTH powder (3000 U/mg) were purchased from Sigma-Aldrich Co (St. Louis, Missouri, USA). All other chemicals were in-house prepared.
A cross-linking reagent solution was prepared by adding 200 µl of BDDE into 9.80 ml of 0.25M NaOH. About 1.20 g of hyaluronic acid HA powder was added to the mixture and allowed to mix thoroughly at room temperature for 60 min. The pH was adjusted at 13 to allow the epoxide ring in BDDE molecules to open and form ether bond with -OH group in HA chains. When reaction was complete, the mixture was neutralized by adding an equivalent amount of 0.1M HCl solution until a pH of approximately 7.0. The mixture was then dialyzed against distilled water for 3 days to remove free BDDE molecules. The resulting mixture was then diluted with distilled water until the final HA concentration became 20 mg/ml. Finally, the cross-linked hydrogel was lyophilized using (Labtech freeze-dryer LFD 5518 model, Daihan Labtech Co) freeze dryer and then stored at 8°C. A native or linear HA solution (20 mg/ml) was also prepared, lyophilized, and stored until the instrumental characterization was carried out.
Equivalent portions from linear and cross-linked HA were analyzed using Bruker Tensor 37; Fourier Transform Infrared Spectroscopy (FTIR) (Ettlingen, Germany). All spectra were recorded between 4000 and 400 cm-1 with a resolution of 4 cm-1 and the data was manipulated using OPUS software.
Equivalent portions from linear and cross-linked HA were obtained and digested by 500 µl of 10.0% (w/v) bovine testicular hyaluronidase BTH enzyme solution (specific activity 3000 U/mg) at 37°C for 2 h. The resulting solutions were centrifuged for 3 min at 3000 rpm using Centurion Scientific centrifuge and the supernatant in each container was collected and diluted 1:50 in purified water. The electro-spray ionization mass spectrometry (ESI-MS) measurements were carried out using Quattro Premier XE mass spectrometer instrument Q-MS (Waters Corporation, Manchester, UK). Analysis was carried out via direct infusion and the experimental conditions were set as follow: capillary voltage 4.0 kV, con voltage (voltage of sampling cone to ionize and direct ions to the mass analyzer) 30 V, dissolvation temperature 150°C and source temperature 100°C. Spectra were acquired in negative ionization mode from m/z 200-1000 with a scan speed of 1 s per scan.
Equivalent portions from linear and cross-linked HA were obtained and digested by 500 µl of 10.0% (w/v) bovine testicular hyaluronidase BTH enzyme solution (specific activity 3000 U/mg) at 37°C for 2 h. The resulting solutions were centrifuged for 3 min at 3000 rpm using Centurion Scientific centrifuge and the supernatant in each container was collected and re-lyophilized. The two samples were then dissolved in Deuterium oxide (D2O) for 1H NMR analysis. Analysis was carried out using 600 MHz 1H Nuclear magnetic resonance spectroscopy (NMR) from Bruker (Zurich, Switzerland).
Equivalent portions were taken from the lyophilized linear and cross-linked HA and coated under vacuum with platinum using an ion sputter prior. The surface structures of the two samples were imaged by scanning electron microscope (SEM) from JEOL (Tokyo, Japan) using the secondary electron imaging (SEI) mode. Both images were captured at similar conditions and magnification level.
In many circumstances, a single analysis is often sufficient for the purposes of the qualitative measurement. However, for a more reliability, the structural characterization was repeated twice for each analytical instrument.
FTIR is a common characterization technique used for linear and cross-linked HA because it allows to determine the type of bond which has been formed during the HA modification [30,31,1]. For instance, [32] observed by using FTIR an additional peak in crosslinked HA at between 2850 cm-1 and 2930 cm-1 which confirmed the presence of alkyl chain of the chemical cross-linker. From other side, the band intensity of the carboxyl groups decreases as the amount of the chemical cross-linker increases [1]. However, [33] stated that no differences can be obtained between the spectra of linear and crosslinked HA except for a band at 1650 cm-1 which can be attributed to the ion exchange of the sodium salt to the acidic form.
In this work, the FTIR spectra as shown in figure 2a for linear HA and figure 2b for cross-linked HA revealed that both samples exhibited almost similar data and there was no appreciable difference that could be noticed. However, by having a closer look at the region between 2800 cm-1 and 3000 cm-1, a little peak can be observed at around 2900 cm-1 in the cross-linked BDDE-HA. This peak was not clearly observed in linear HA which represented the C-H stretching in the chemical cross-linker. A second characteristic peak at about 3343 cm-1 commonly representing the hydroxyl group was observed in linear HA and cross-linked BDDE-HA. However, the peak area in linear HA was larger than its counterpart in cross-linked BDDE-HA indicating that the amount of hydroxyl groups after cross-linking became less.
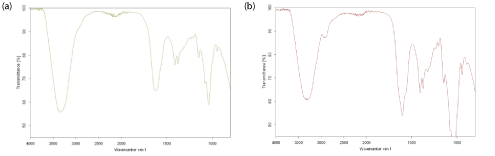
Figure 2: (a) FTIR spectra of lyophilized linear HA sample (b) FTIR spectra of cross-linked BDDE-HA obtained at 25°C between 4000 and 400 cm-1.
This means that a chemical modification could have happened between HA chains and BDDE molecules via hydroxyl groups to form a new interconnected network. Theoretically, there are four alcohols reactive sites per unit of HA and two epoxide groups in one BDDE molecule. The relative preference of epoxide groups to react with hydroxyl groups depends on reaction conditions. As we stated, under alkaline conditions, the BDDE molecules target the reactive hydroxyl groups in linear HA to form ether bonds. If two groups in two adjacent HA chains are covalently blocked with BDDE epoxides, the total hydroxyl groups will decrease. This account for their appearance with smaller downward peak than in non-modified or linear HA.
One small peak at about 1300 cm-1 appearing in the cross-linked BDDE-HA but not in linear HA confirmed the successful crosslinking process. This peak could be assigned to the formation of another bond between HA chains and BDDE molecules. In fact, when HA cross-linking is carried out at high pH value (above the pKa value of the hydroxyl group), the hydroxyl groups of HA become almost deprotonated. Hence, the epoxide groups of BDDE react preferentially with the hydroxyl groups of HA to form stable ether bonds [1].
Finally, these three characteristic peaks could differentiate between linear and cross-linked BDDE-HA structures.
Due to the viscoelastic properties and complex mixture of larger oligosaccharides generated by hyaluronic acid degradation, the application of ESI-MS for HA oligosaccharides is still challenging [11]. A one study performed by [34] using ESI-ion trap mass spectrometer pointed out that under ESI conditions, the HA molecules are multiply charged and the spectra are more difficult to interpret. In addition, the ESI-tandem MS used negative ionization mode and showed a loss of one molecule N-acetyl glucosamine as [M-H-H2O], m/z=202 for the even numbered oligomers, while odd-numbered oligomers split off glucuronic acid as [M-H-H2O], m/z=175.
In this work, the linear HA and cross-linked BDDE-HA showed various oligosaccharide fragmentation pattern with different relative intensities and chain length ranged from the basic unit of hyaluronic acid (m/z 378) to a greater than 16-mers. The oligosaccharides of cross-linked BDDE-HA exhibited a different mapping spectra and different charge state distribution profile than linear HA. Based on data displayed in figure 3a, most of linear HA ions were observed at lower m/z range or below than m/z 400 (with the exception of few peaks observed at higher m/z range) and with much larger peak intensities than those observed in the counterpart spectra of crosslinked BDDE-HA.
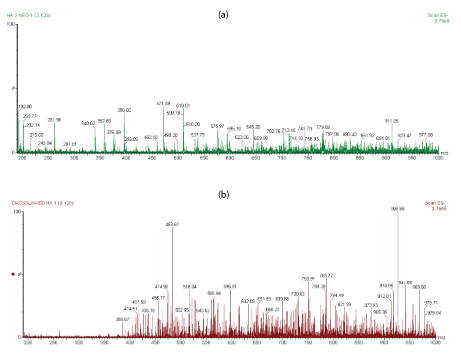
Figure 3: (a) The ESI-MS profiles of linear HA sample (b) ESI-MS spectra of cross-linked HA-BDDE obtained via direct infusion with 4.0 kV capillary voltage and 30 v con voltage.
The ions of cross-linked BDDE-HA, figure 3b became more abundant after m/z 400 due to the slow degradation rate of cross-linked BDDE-HA which had a higher resistance toward enzymatic digestion than linear HA. For instance, the peaks at m/z 396, m/z 192.8, and m/z 201.89 appeared with very low peak intensity in the cross-linked sample with respect to the linear HA.
For a more comparison, figures 4a,4b shows the extended m/z profiles of linear HA and cross-linked BDDE-HA respectively from m/z 200 to m/z 300, whereas figures 5a,5b shows the extended m/z profiles of the same samples from m/z 300 to m/z 400. The peak at m/z 396 represented the basic disaccharide unit of hyaluronic acid companied with a water molecule ([GlcUA-GlcNAc]+H2O). Also, the peaks at m/z 192.8 and m/z 201.89 were attributed to glucoronic acid (GlcA+H2O) and N-acetyl-D-glucosamine (GlcNAc-H2O) respectively.
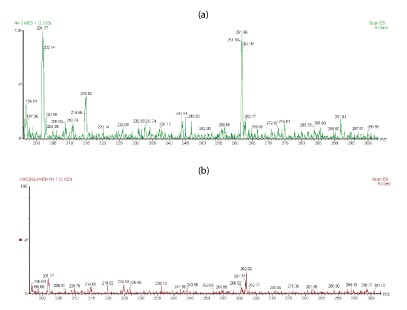
Figure 4: (a) Extended m/z profile of linear HA from m/z 200 to m/z 300 (b) Extended m/z profile of cross-linked HA-BDDE from m/z 200 to m/z 300.
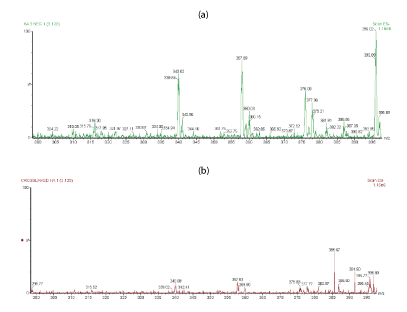
Figure 5: (a) Extended m/z profile of linear HA from m/z 300 to m/z 400 (b) Extended m/z profile of cross-linked HA-BDDE from m/z 300 to m/z 400.
Although the enzyme cleaves the 1,4-linkages between N-acetyl-Dglucosamine (GlcNAc) and glucoronic acid (GlcA) yielding differentsized oligomers with N-acetyl-D-glucosamine at the reducing terminal and unsaturated uronic acid (ΔUA) at the non-reducing terminal (even-numbered oligosaccharides) and sometime fragments with uronic acid UA at both sides (odd-numbered oligosaccharides), the high molecular weight ions-in our method-were difficult to be identified due to the fragmentation and collisional activation which are usually experienced during the ESI-MS analysis.
In addition, we observed that the high molecular weight ions were greatly influenced by method conditions and mass spectrometric parameters. For example, any change in cone voltage or dissolvation temperature produces different fragmentation pattern. In contrast, the low molecular weight ions were easily defined and they were in good agreement with the theoretical ion species of HA degradation products.
Despite that the pretreatment of polymers in H1 NMR analysis is challenging particularly when viscous polymers are considered [1], NMR proved to be a powerful technique for characterizing the linear and chemically cross-linked HA. A one previous study carried out by La Gatta A, et al. [35] reported that a signal around 1.6 ppm observed in the cross-linked HA due to the presence (CH2)2 moiety of the BDDGE molecule. Similar data were almost acquired by Wende FJ, et al. [36] for using H1 NMR. Twwo main peaks were identified in crosslinked BDDE-HA: the N-acetyl signal (CH3) from HA at around 2.1 ppm and the (CH2) moiety from BDDE at around 1.7 ppm. Figure 6 shows a typical 1H NMR spectra comparing linear HA and cross-linked BDDE-HA hydrogel carried out by Guarise, et al. [37].
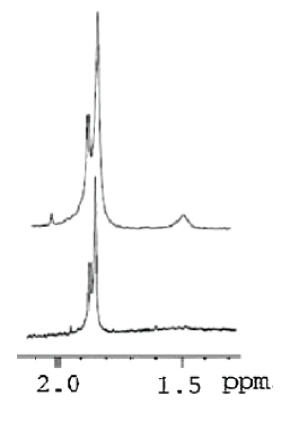
Figure 6: H1 NMR spectra of linear HA (lower) and BDD-HA (higher) [37].
According to the present work, the NMR spectra as shown in figure 7a for linear HA and figure 7b for cross-linked HA, there are two main characteristic peaks: peak 1 at about 1.9 ppm which appeared in both samples and peak 2 at about 1.5 ppm which only appeared in the cross-linked BDDE-HA. Peak 1 was mainly attributed to the presence of acetyl glucosamine N-CH3 group which is found in HA backbone structure. Peak 2 represented the -(CH2) group which is found in BDDE molecules. These findings unambiguously confirmed that the cross-linked BDDE-HA exhibited different NMR spectra from linear HA and proved that HA chains in BDDE-HA had been chemically reacted with the cross-linker.
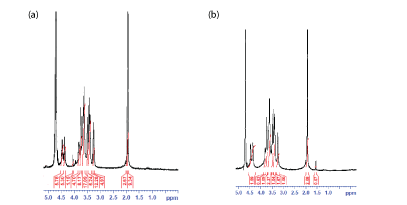
Figure 7: (a) 1H NMR spectra of linear HA sample (b) 1HNMR spectra of cross-linked BDDE-HA sample.
In the NMR analysis of cross-linked HA polysaccharides, integrating the amount of -(CH2) group at 1.5 ppm with regard to the amount of acetyl glucosamine N-CH3 at 1.9 ppm is commonly practiced to estimate the total degree of chemical modification occurred in linear HA [8,29]. The peak of -(CH2) group in BDDE has the advantage of not being superimposed with the peaks of the linear HA allowing easy evaluation of the total degree of chemical modification in HA chains.
Most of previous SEM studies indicated that linear HA has a fibrous and irregular structure whereas the cross-linked HA has a highly porous and sheet-like surface structure [1]. The degree of crosslinking also largely affects the morphological structure and degree of interconnectivity of cross-linked BDDE-HA with pore diameters ranging from a few microns to around 100 μm [38].
The data of SEM illustrated different micro-pours structures for linear HA and cross-linked BDDE-HA. The micro-porosity structure of cross-linked BDDE-HA, figure 7b was more homogenous and showed better uniformity than the micro-porosity structure of linear HA, figure 7a. Comparing to the linear HA matrix which showed very large and open pores, the pores of cross-linked BDDE-HA were very small and had narrow pore-size distribution ranged from less than 1 µm to around 10 µm. In fact, the property of homogeneity coupled with narrow pore size distribution in the cross-linked BDDE-HA holds a great promise and interest in biomedical research particularly for tissue engineering and drug delivery.
Although the SEM images did not prove the occurrence of chemical linkage in the cross-linked BDDE-HA via distinctive peaks as concluded in FTIR, ESI-MS and NMR, however, the rigid and cohesive matrix observed throughout BDDE-HA surface micro-structure in comparison to the very weak scaffold observed in linear HA verified this conclusion. This indicates that the influence of chemical reaction between HA chains and BDDE was quite significant and produced a noticeable and well-interconnected scaffold that exhibits higher resistance against enzymatic degradation than linear HA (Figures 8a,8b).
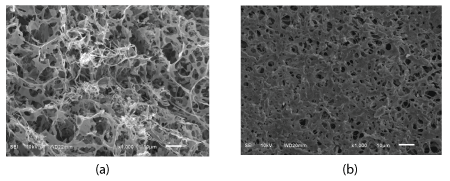
Figure 8: (a) SEM image of linear HA sample (b) SEM image of cross-linked BDDE-HA sample obtained at identical magnification conditions (x1000, SEI and 10kv).
The aim of this work was to characterize linear and cross-linked BDDE-HA using FTIR, ESI-MS, H1 NMR and SEM. The FTIR spectra showed an additional little peak at around 2900 cm-1 in cross-linked BDDE-HA. At the same time, the peak intensity of hydroxyl groups at about 3343 cm-1 in the cross-linked BDDEHA was much less than its counterpart in native HA. The ESI-MS analysis was more characteristic by detecting different mass spectra profiles for linear and cross-linked BDDE-HA. The molecular ions of linear HA were more abundant below 400 m/z compared to the cross-linked BDDE-HA that showed ions with higher molecular weights. NMR is a powerful technique for characterization of linear HA and cross-linked BDDE-HA. It showed a distinctive peak at 1.5 ppm for BDDE-HA that was not shown in linear HA. Data obtained from SEM showed that the cross-linked BDDE-HA surface microstructure had smaller pore-size and it was more regularly distributed than linear HA.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Al-Sibani M, Al-Harrasi A, Neubert RHH (2018) Characterization of Linear and Chemically Cross-linked Hyaluronic acid using Various Analytical Techniques Including FTIR, ESI-MS, H1 NMR, and SEM. J Biochem Analyt Stud 3(1): dx.doi.org/10.16966/2576-5833.115
Copyright: © 2018 Al-Sibani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access