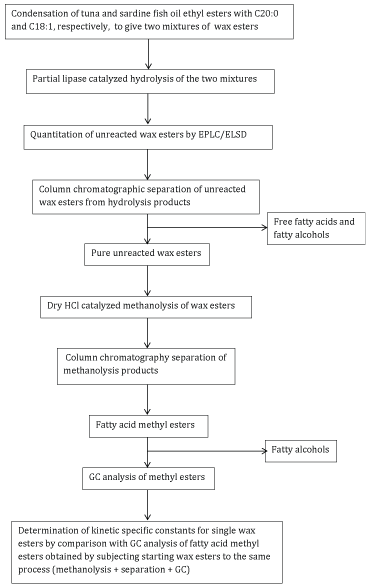
Scheme 1

Carmine Capozzoli1 Guido Galliani2* Francesco Ricot1 Alberto Terraneo1
1Actygea srl, Insubrias BioPark, Via Roberto Lepetit, 34, 21040 Gerenzano VA, Italy*Corresponding author: Guido Galliani, Gleaner srls, via San Francesco, 5, 01010, Farnese VT, Italy, Tel: 0039 347 49 33 373; E-mail: guidogalliani8@gmail.com
Wax esters are esters of fatty acids with fatty alcohols and represent a lipid class showing two different descriptions when it comes to their metabolism and absorbance in humans. On one side, they are described as non-digestible materials, such as for instance in the case of spermaceti and jojoba oil. On the other side, they are major constituents of roe and, as such, they are currently consumed as a part of traditional food all over the world. Moreover, an oil rich in wax ester from Calanus finmarchicus has been recently launched commercially. The present paper describes competitive kinetics of hydrolysis of two different wax esters catalysed by three different lipases, namely porcine pancreas lipase, Candida cylindracea lipase, and Candida antarctica lipase. Wax esters were synthesized by condensing ethyl ester from tuna and sardine oil, respectively, with behenyl alcohol and oleyl alcohol, respectively. Multiple regression analysis showed that apparent constants kapp are related to both the length and the number of double bonds of the acid moiety. The higher these values, the higher the hydrolysis rate. This is not affected by the fatty alcohol moiety. Similar results were obtained from the three lipases. For porcine pancreas lipase, the correlation takes the form krel hydrolysis=0.0222 nCacid+0.0571 n∆acid, where n∆acid is the number of carbon atoms in the fatty chain and n∆acid is the number of C-C double bonds in the same chain. Results will be discussed in order to clarify differences in digestibility of different wax esters.
Wax esters; Digestibility; Lipases; Hydrolysis; Kinetics; Polyunsaturated fatty acids
Wax esters are a class of lipids, featuring esters of fatty acids with fatty alcohols. Until not so long ago, wax esters had an overall bad reputation. Spermaceti, jojoba oil, orange roughy oil are typical examples of wax esters, known insofar that they are used topically, mostly for cosmetic purposes. However, it was equally well known that these products should never be ingested, under penalty of very unpleasant consequences, such as nausea, diarrhoea, and steatorrhea. Several papers were dedicated to understanding how wax esters are absorbed and digested in animals [1-8]. However, a statement on wax esters structure-reactivity relationships in the presence of lipase was still missing. We thought that investigating on any possible structure-reactivity relationship could be useful in understanding some contradictory observations reported below. Some years ago, we started a program in order to understand some rather conflicting data. It was known that roe (fish eggs) is consumed as a food since time immemorial. This occurs either on roe as is (caviar, lumpfish roe) or after some treatment (Italian bottarga, Greek taramosalata, Japanese karasumi, Scandinavian Lysekils kaviar). Moreover, it was known that wax esters are an important constituent of roe, in some instances the majority component. In spite of this, no concern had ever been raised as to edibility or side effects of such products. The first results in analysing wax esters from several roe-based foods showed that polyunsaturated fatty acids were a very important component of the acidic moiety [9,10]. Successive results from other research groups confirmed our original results [11-13]. These findings partially supported substantiating the launch of a new commercial product, Calanus® oil, extracted from copepod Calanus finmarchicus, rich in wax esters and in omega-3 polyunsaturated acids [14].
From these facts, it could be inferred that the term wax ester is just a rough chemical definition. Within this class of lipids different molecules are included. Some of them are essentially saturated molecules, likely intended to assist buoyancy and to store energy. Other molecules represent a more complex metabolism, not yet fully understood. According to this hypothesis, differences should be likely reflected in the metabolic fate when wax esters are ingested.
The aims of this work were:

Scheme 1
Preparation of a wax ester from docosanol and ethyl esters from tuna oil (Product WE-C22:0-TU): In a 100 mL rounded bottom flask, 5 g of 90% docosanol (commercial name Lanette 22) were mixed with 5 g of dry ethyl ester from ethanolysis of deacidified tuna oil. 0.25 mL of a 22% solution of sodium ethylate in ethanol were added. The flask was connected to vacuum (20 mm/Hg) and the mixture was heated under stirring at 90°C, whereby evolution of ethanol started vigorously. After keeping this temperature for 12 hours, vacuum was disconnected and the mixture was cooled at 20°C and dissolved in 25 mL of chloroform. The solution was then washed with into 50 mL of 7% solution (w/w) of citric acid, separated and washed again with 50 mL of water. Finally, the oily phase was evaporated and dried at 80°C under reduced pressure on a Rotavapor. The mixture was analysed by TLC (thin layer chromatography) silica gel plates, eluting system: n-hexane/ethyl acetate 19:1, stained by spraying phosphomolibdic reagent and heating at 100°C for 10 minutes). TLC showed three spots at Rf 0.79, 0.55, and 0.13, corresponding to wax esters, ethyl esters, and fatty alcohols, respectively, the spot due to ethyl esters being much less visible than the other two. Pure wax esters were prepared by column chromatography according to the following procedure. The raw product was dissolved in a mixture of 30 mL of n-hexane/ethyl acetate 39:1 v/v. The solution was loaded on a column (internal diameter 7.0 cm, height 60.0 cm) filled with 500 g of silica gel (high purity grade, Daisil Grade 636, pore size 60 Å, 35 – 60 mesh particle size, catalogue number 236802, Sigma Aldrich) and conditioned with a 5.0 L of mixture n-hexane/ethyl acetate 39:1 (v/v). The mixture was then eluted by the same solvent used for conditioning the column, by collecting 100 mL fractions. Fractions containing pure wax esters (TLC) were collected and evaporated under reduced pressure. Ca. 7 g of pure wax ester were obtained (WE-22:0-TU).
Preparation of a wax ester from oleyl alcohol and ethyl esters from fish oil (Product WE-C18:1-FI): The same procedure was repeated by using 10 g of 90% oleyl alcohol (commercial name Rofanol) and 10 g of dry ethyl ester from ethanolysis of deacidified sardine oil. Ca. 7 g of pure wax ester was obtained (WE-18:1-FI).
Hydrolysis of wax esters by porcine pancreas lipase: The following reagents were prepared: A: Wax Ester; B: 3 M Sodium Chloride Solution (NaCl) (100 mL in deionized water using Sodium Chloride); C: 1.5% (weight/volume) Sodium Taurocholate Solution (25 mL in deionized water using Taurocholic Acid, Sodium Salt, Sigma Prod. No. T-4009); D: 75 mM Calcium Chloride Solution (CaCl2 ) (25 mL in deionized water using Calcium Chloride, Dihydrate); E: 10 mM Sodium Hydroxide Solution-Standardized (NaOH) (50 mL in cold deionized water using Sodium Hydroxide); F: 5 mM Calcium Chloride Solution (25 mL in deionized water using Calcium Chloride, Dihydrate); G: Lipase Enzyme Solution (Sigma Aldrich Lipase type II, code: L3126-25G, 20% protein, 500 U/g protein. The solution was prepared immediately before use, as a suspension containing 500 mg of Lipase in 2.0 mL of cold Reagent F. This amount corresponds to 250 U of lipase). For hydrolysis, the following procedure was used: a reaction mixture was prepared by pipetting the following reagents into a suitable container: deionized water 750 µL, Reagent A 350 mg, Reagent B 210 µL, Reagent C 210 µL, Reagent D 100 µL. The mixture was mixed by swirling and adjusted to pH 8.0 at 37°C with Reagent E. After equilibrating at 37°C, 2.0 mL of Reagent G were added. Stirring was stopped after 4 hours. The mixture was diluted with 20 mL of water and extracted with 20 mL of chloroform. The chloroform fraction was filtered on a paper filter, washed with 20 mL of water, and driedunder reduced pressure on Rotavapor at 80°C. TLC under the same conditions described before (n-hexane-ethyl acetate 19:1) showed the spots due to non-reacted wax ester, fatty alcohols, and a spot at Rf 0.06, corresponding to free fatty acids.
Hydrolysis of wax esters by Candida cylindracea lipase: 200 mg of the wax ester were dissolved in 20 mL of n-hexane. Lipase OF (trademark) was used (Sangyo, from Candida cylindracea, activity: 360,000 U/g). 100 mg of lipase, corresponding to 36.000 U, were dissolved in 20 mL of water. pH was 6.5. The two solutions were mixed and the two-phase system was stirred at 37°C for 18 hours. 60 mL of water were then added and the mixture was heated at 80°C. After cooling at 30°C, the mixture was extracted with chloroform (2 × 20 mL). The organic phases were collected, washed with water (2 × 20 L), filtered on a paper filter to eliminate any emulsified material, and dried at 80°C under reduced pressure till constant weight.
Hydrolysis of wax esters by Candida antarctica lipase: 200 mg of the wax ester were dissolved in 20 mL of n-hexane. Lipase NOVOCOR AD L (Trademark, Novozymes, from Candida Antarctica, aqueous solution, activity 6,000 U/g) 100 mg of lipase (600 U) were diluted in 20 mL of water. The two solutions were mixed and the two-phase system was stirred at 37°C for 18 hours. pH was 6.7. 60 mL of water were then added and the mixture was heated at 80°C. After cooling at 30°C, the mixture was extracted with chloroform (2 × 20 mL). The organic phases were collected, washed with water (2 × 20 L), filtered on a paper filter to eliminate any emulsified material, and dried at 80°C under reduced pressure till constant weight.
Determination of the non-reacted wax esters: The method used has been described for the separation of polar and nonpolar lipids on the same HPLC column [15]. A HPLC (high performace liquid chromatography) Hewlett Packard Series 1050 was used, endowed with an Evaporative Light Scattering Detector (ELSD) Bischoff 3000 (Leonburg, Germany). Separation used a Chromolith® Performance column (Millipore, Germany) silica, 100 mm length, 4.6 mm ID. For each oil sample, a first solution was prepared in chloroformmethanol 2:1 (v/v), at an exact concentration of 200 µg/mL. Solvents used were: 2,2,4-trimethylpentane, ethyl acetate, and 2-propanol all Chromasolv, from Sigma-Aldrich (Saint Louis, USA); acetone LiChrosolv®, ethanolamine MSynth®, both from Merck (Darmstadt, Germany); acetic acid puriss. ACS reagent from Honeywell (Seelze, Germany). Results were compared with calibration curves for WE, previously prepared. A typical separation pattern is reported in figure 1.
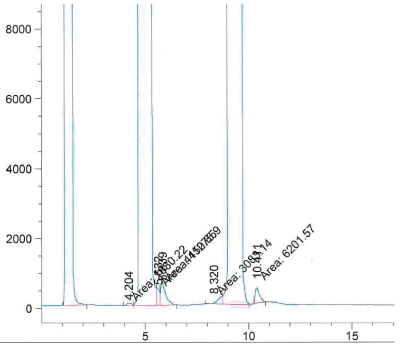
Figure 1: Typical HPLC separation of wax ester (major peak on the left), fatty alcohols (major central peak) and free fatty acids (major peak on the right.
Separation of non-reacted wax ester: After the HPLC analysis, 150 mg of the reaction mixture were dissolved in 2.0 mL of n-hexane. This solution was applied on the top of a column of silica-gel (13.0 g) conditioned with 250 mL of a mixture n-hexane/ethyl acetate 19:1 (v/v). The mixture was then eluted by the same solvent mixture used for conditioning. 12 mL fractions were collected. Fractions showing in TLC the spot of wax esters were pooled together and evaporated under reduced pressure.
Analysis of the fatty acid composition of initial and unreacted wax esters after hydrolysis: The same procedure was applied on both the purified product synthesized as described before and to residual wax esters not hydrolyzed by lipases. The analysis was performed according to the following procedure: either ca. 150 mg of the pure initial wax esters or the unreacted wax esters purified according to the preceding procedure were suspended in 5 mL of 3 N dry hydrogen chloride in methanol, obtained by reacting the due amount of acetyl chloride in methanol. The suspension was prepared in a screw cap vial that was then closed and placed in a bath at 90°C for three hours and occasionally stirred. After this time, the solution obtained was cooled at 60°C and poured into 30 mL of water. The mixture thus obtained was extracted with chloroform (2 × 20 mL). The organic phases were collected, washed with water (2 × 20 mL), and evaporated under reduced pressure at 80°C till constant weight. The residue was dissolved in 2.0 mL of n-hexane. This solution was applied on the top of a column of silica-gel (13.0 g) conditioned with 250 mL of a mixture n-hexane/ethyl acetate 19:1 (v/v). The mixture was then eluted by the same solvent mixture used for conditioning. 12 mL fractions were collected. Fractions containing methyl esters were put together and evaporated under reduced pressure. Fatty acid methyl esters were then analysed by GC. Column: Agilent CP-WAX 52 CB. Length 25 m, 0.25 mm ID. 0.20 µm film thickness. Carrier: helium. Injector temperature: 250°C. FID detector temperature: 270°C Gradient: hold 1 minute at 90°C, then raise temperature at a rate of 30°C/min to 150°C, then raise temperature at a rate of 3°C/min to 225°C, finally hold this temperature for 7 minutes. Injection volume 1 µL of a solution of the sample in isooctane, at a concentration of 150 µg/mL. Values obtained from three analyses gave area percentages within an error of ± 1.5% calculated over total areas. A typical chromatogram is shown in figure 2.
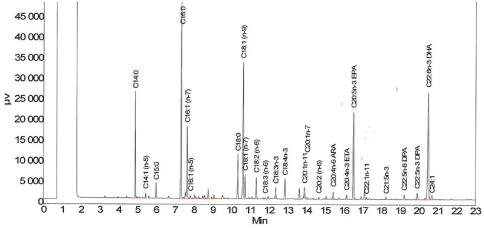
Figure 2: Typical chromatogram of wax ester from tuna oil and docosanol
GC analysis of the acid moiety of both unreacted and partially hydrolysed wax esters showed nineteen major peaks, each of them was higher than 3% of the total area. Other minor peaks sum up to less than 15% of the total area. Absolute areas (in mV*sec) of the nineteen major peaks were added up to give a total sum S. Each area of the major peaks was normalized by dividing its actual area by the sum S, expressed as a percentage. Tables 1-6 show in the first column these nineteen major fatty acids. Second columns report the normalized area percentages of these fatty acids for starting wax esters. Third columns report the normalized area percentages of the same fatty acids found in unreacted wax esters after a due reaction time. This was 18 hours in the case of the two microbial lipases and 4 hours in the case of porcine pancreas lipase.
| STARTNG WE: WE-C22:0-TU residual WE: 0,71 | LIPASE: CANIDA CYLINDRACEA (LIPASE OF) | |||||
| FAME | area % t0 | area % 18 h | corrected area % 18 h | delta area % | kapp =delta/area t0 | kapp rel |
| C14:0 | 6,1 | 7 | 5 | 1,1 | 0,18 | 0,2 |
| C14:1 (n-5) | 0,4 | 0,3 | 0,2 | 0,2 | 0,5 | 0,7 |
| C15:0 | 1 | 1,2 | 0,9 | 0,1 | 0,1 | 0,1 |
| C16:0 | 18,1 | 21,6 | 15,3 | 2,8 | 0,15 | 0,2 |
| C16:1 (n-7) | 6,1 | 6,6 | 4,7 | 1,4 | 0,23 | 0,3 |
| C18:0 | 4,3 | 5 | 3,6 | 0,7 | 0,16 | 0,2 |
| C18:1 (n-9) | 14 | 15,3 | 10,9 | 3,1 | 0,22 | 0,3 |
| C18:1 (n-7) | 2,2 | 2,3 | 1,6 | 0,6 | 0,27 | 0,4 |
| C18:2 (n-6) | 2,1 | 2,2 | 1,6 | 0,5 | 0,24 | 0,3 |
| C18:3 (n-3) | 1,2 | 1,3 | 0,9 | 0,3 | 0,25 | 0,3 |
| C18:4 (n-3) | 2,5 | 2,4 | 1,7 | 0,8 | 0,32 | 0,4 |
| C20:0 | 1,1 | 1,2 | 0,9 | 0,2 | 0,18 | 0,2 |
| C20:1 (n-11) | 1,3 | 0,9 | 0,6 | 0,7 | 0,54 | 0,7 |
| C20:4 (n-6) | 1 | 1 | 0,7 | 0,3 | 0,3 | 0,4 |
| C20:4 (n-3) | 0,9 | 0,7 | 0,5 | 0,4 | 0,44 | 0,6 |
| C20:5 (n-3) | 13,1 | 10,4 | 7,4 | 5,7 | 0,44 | 0,6 |
| C22:5 (n-6) | 2,4 | 0,8 | 0,6 | 1,8 | 0,75 | 1 |
| C22:5 (n-3) | 2,8 | 1,1 | 0,8 | 2 | 0,71 | 0,9 |
| C22:6 (n-3) | 19,3 | 18,8 | 13,3 | 6 | 0,31 | 0,4 |
Table 1: Analysis of fatty acids in unreacted wax esters from tuna oil and docosanol. Lipase from Candida cylindracea. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 18 hours. Conversion was 100–71=29%. Further columns show data elaboration.
| STARTNG WE: WE-C22:0-TU residual WE: 0,5 | LIPASE: PORCINE PANCREAS LIPASE | |||||
| FAME | area % t0 | area % 4 h | corrected area % 4 h | delta area % | kapp =delta/area t0 | kapp rel |
| C14:0 | 6,1 | 9,6 | 4,8 | 1,3 | 0,21 | 0,2 |
| C14:1 (n-5) | 0,4 | 0,3 | 0,2 | 0,2 | 0,5 | 0,5 |
| C15:0 | 1 | 1 | 0,5 | 0,5 | 0,5 | 0,5 |
| C16:0 | 18,1 | 27,5 | 13,8 | 4,3 | 0,24 | 0,3 |
| C16:1 (n-7) | 6,1 | 11,3 | 5,7 | 0,4 | 0,07 | 0,1 |
| C18:0 | 4,3 | 6,3 | 3,2 | 1,1 | 0,26 | 0,3 |
| C18:1 (n-9) | 14 | 14,8 | 7,4 | 6,6 | 0,47 | 0,5 |
| C18:1 (n-7) | 2,2 | 4,4 | 2,2 | 0 | 0 | 0 |
| C18:2 (n-6) | 2,1 | 1,7 | 0,9 | 1,2 | 0,57 | 0,6 |
| C18:3 (n-3) | 1,2 | 0,7 | 0,4 | 0,8 | 0,67 | 0,7 |
| C18:4 (n-3) | 2,5 | 1,9 | 1 | 1,5 | 0,6 | 0,6 |
| C20:0 | 1,1 | 1,2 | 0,6 | 0,5 | 0,45 | 0,5 |
| C20:1 (n-11) | 1,3 | 0,3 | 0,2 | 1,1 | 0,85 | 0,9 |
| C20:4 (n-6) | 1 | 0,8 | 0,4 | 0,6 | 0,6 | 0,6 |
| C20:4 (n-3) | 0,9 | 0,6 | 0,3 | 0,6 | 0,67 | 0,7 |
| C20:5 (n-3) | 13,1 | 10,5 | 5,3 | 7,8 | 0,6 | 0,6 |
| C22:5 (n-6) | 2,4 | 0,2 | 0,1 | 2,3 | 0,96 | 1 |
| C22:5 (n-3) | 2,8 | 0,9 | 0,5 | 2,3 | 0,82 | 0,9 |
| C22:6 (n-3) | 19,3 | 5,9 | 3 | 16,3 | 0,84 | 0,9 |
Table 2: Analysis of fatty acids in unreacted wax esters from tuna oil and docosanol. Porcine pancreas lipase. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 4 hours. Conversion was 100–50=50%. Further columns show data elaboration (see Discussion).
| STARTNG WE: WE-C22:0-TU residual WE: 0,65 | LIPASE: CANDIDA ANTARCTICA | |||||
| FAME | area % t0 | area % 4 h | corrected area % 18 h | delta area % | kapp =delta/area t0 | kapp rel |
| C14:0 | 6,1 | 8,9 | 5,8 | 0,3 | 0,05 | 0,1 |
| C14:1 (n-5) | 0,4 | 0,5 | 0,3 | 0,1 | 0,25 | 0,3 |
| C15:0 | 1 | 1,5 | 1 | 0 | 0 | 0 |
| C16:0 | 18,1 | 27,7 | 18 | 0,1 | 0,01 | 0 |
| C16:1 (n-7) | 6,1 | 9,3 | 6 | 0,1 | 0,02 | 0 |
| C18:0 | 4,3 | 6,3 | 4,1 | 0,2 | 0,05 | 0,1 |
| C18:1 (n-9) | 14 | 20,6 | 13,4 | 0,6 | 0,04 | 0 |
| C18:1 (n-7) | 2,2 | 3 | 2 | 0,2 | 0,09 | 0,1 |
| C18:2 (n-6) | 2,1 | 2,2 | 1,4 | 0,7 | 0,33 | 0,3 |
| C18:3 (n-3) | 1,2 | 0,9 | 0,6 | 0,6 | 0,5 | 0,5 |
| C18:4 (n-3) | 2,5 | 0,9 | 0,6 | 1,9 | 0,76 | 0,8 |
| C20:0 | 1,1 | 1,5 | 1 | 0,1 | 0,09 | 0,1 |
| C20:1 (n-11) | 1,3 | 0,3 | 0,2 | 1,1 | 0,85 | 0,9 |
| C20:4 (n-6) | 1 | 0,5 | 0,3 | 0,7 | 0,7 | 0,7 |
| C20:4 (n-3) | 0,9 | 0 | 0 | 0,9 | 1 | 1 |
| C20:5 (n-3) | 13,1 | 4,7 | 3,1 | 10 | 0,76 | 0,8 |
| C22:5 (n-6) | 2,4 | 0,2 | 0,1 | 2,3 | 0,96 | 1 |
| C22:5 (n-3) | 2,8 | 0,4 | 0,3 | 2,5 | 0,89 | 0,9 |
| C22:6 (n-3) | 19,4 | 5,9 | 3,8 | 15,6 | 0,8 | 0,8 |
Table 3: Analysis of fatty acids in unreacted wax esters from tuna oil and docosanol. Lipase from Candida antarctica. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 18 hours. Conversion was 100 65=35%. Further columns show data elaboration (see Discussion).
| STARTNG WE: WE-C18:1-FI residual WE: 0,75 | LIPASE: CANIDA CYLINDRACEA (LIPASE OF) | |||||
| FAME | area % t0 | area % 18 h | corrected area % 18 h | delta area % | kapp =delta/area t0 | kapp rel |
| C14:0 | 9,6 | 10 | 7,5 | 2,1 | 0,22 | 0,3 |
| C14:1 (n-5) | 0,9 | 1 | 0,8 | 0,1 | 0,11 | 0,1 |
| C15:0 | 1,2 | 0,9 | 0,7 | 0,5 | 0,42 | 0,5 |
| C16:0 | 21,6 | 25,5 | 19,1 | 2,5 | 0,12 | 0,1 |
| C16:1 (n-7) | 11,4 | 11 | 8,3 | 3,1 | 0,27 | 0,3 |
| C18:0 | 4 | 5,1 | 3,8 | 0,2 | 0,05 | 0,1 |
| C18:1 (n-9) | 12,5 | 12,7 | 9,5 | 3 | 0,24 | 0,3 |
| C18:1 (n-7) | 3,9 | 4,1 | 3,1 | 0,8 | 0,21 | 0,3 |
| C18:2 (n-6) | 1,6 | 1,6 | 1,2 | 0,4 | 0,25 | 0,3 |
| C18:3 (n-3) | 0,9 | 0,7 | 0,5 | 0,4 | 0,44 | 0,5 |
| C18:4 (n-3) | 2,7 | 2,3 | 1,7 | 1 | 0,37 | 0,4 |
| C20:0 | 0,6 | 0,8 | 0,6 | 0 | 0 | 0 |
| C20:1 (n-11) | 1,8 | 0,4 | 0,3 | 1,5 | 0,83 | 1 |
| C20:4 (n-6) | 1,1 | 1 | 0,8 | 0,3 | 0,27 | 0,3 |
| C20:4 (n-3) | 0,8 | 0,7 | 0,5 | 0,3 | 0,38 | 0,5 |
| C20:5 (n-3) | 15,4 | 13,1 | 9,8 | 5,6 | 0,36 | 0,4 |
| C22:5 (n-6) | 0,6 | 0,3 | 0,2 | 0,4 | 0,67 | 0,8 |
| C22:5 (n-3) | 0,9 | 0,4 | 0,3 | 0,6 | 0,67 | 0,8 |
| C22:6 (n-3) | 9,1 | 8,5 | 6,4 | 2,7 | 0,3 | 0,4 |
Table 4: Analysis of fatty acids in unreacted wax esters from sardine oil and oleyl alcohol. Lipase from Candida cylindracea. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 18 hours. Conversion was 100–75=25%. Further columns show data elaboration (see Discussion).
| STARTNG WE: WE-C18:1-FI residual WE: 0,54 | LIPASE: PORCINE PANCREAS LIPASE | |||||
| FAME | area % t0 | area % 4 h | corrected area % 4 h | delta area % | kapp =delta/area t0 | kapp rel |
| C14:0 | 9,6 | 9,6 | 5,2 | 4,4 | 0,46 | 0,5 |
| C14:1 (n-5) | 0,9 | 0,3 | 0,2 | 0,7 | 078 | 0,9 |
| C15:0 | 1,2 | 1 | 0,5 | 0,7 | 0,58 | 0,7 |
| C16:0 | 21,6 | 27,5 | 14,9 | 6,7 | 0,31 | 0,3 |
| C16:1 (n-7) | 11,4 | 11,3 | 6,1 | 5,3 | 0,46 | 0,5 |
| C18:0 | 4 | 6,3 | 3,4 | 0,6 | 0,15 | 0,2 |
| C18:1 (n-9) | 12,5 | 14,8 | 8 | 4,5 | 0,36 | 0,4 |
| C18:1 (n-7) | 3,9 | 4,4 | 2,4 | 1,5 | 0,38 | 0,4 |
| C18:2 (n-6) | 1,6 | 1,7 | 0,9 | 0,7 | 0,44 | 0,5 |
| C18:3 (n-3) | 0,9 | 0,7 | 0,4 | 0,5 | 0,56 | 0,6 |
| C18:4 (n-3) | 2,7 | 1,9 | 1 | 1,7 | 0,63 | 0,7 |
| C20:0 | 0,6 | 1,2 | 0,6 | 0 | 0 | 0 |
| C20:1 (n-11) | 1,8 | 0,3 | 0,2 | 1,6 | 0,89 | 1 |
| C20:4 (n-6) | 1,1 | 0,8 | 0,4 | 0,7 | 0,64 | 0,7 |
| C20:4 (n-3) | 0,8 | 0,6 | 0,3 | 0,5 | 0,63 | 0,7 |
| C20:5 (n-3) | 15,4 | 10,5 | 5,7 | 9,7 | 0,63 | 0,7 |
| C22:5 (n-6) | 0,6 | 0,2 | 0,1 | 0,5 | 0,83 | 0,9 |
| C22:5 (n-3) | 0,9 | 0,9 | 0,5 | 0,4 | 0,44 | 0,5 |
| C22:6 (n-3) | 9,1 | 5,9 | 3,2 | 5,9 | 0,65 | 0,7 |
Table 5: Analysis of fatty acids in unreacted wax esters from sardine oil and oleyl alcohol. Porcine pancreas lipase. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 4 hours. Conversion was 100–54=46%. Further columns show data elaboration (see Discussion).
| STARTNG WE: WE-C18:1-FI residual WE: 0,63 | LIPASE: CANDIDA ANTARCTICA | |||||
| FAME | area % t0 | area % 18 h | corrected area % 4 h | delta area % | kapp =delta/area t 0 | kapp rel |
| C14:0 | 9,6 | 10,2 | 6,4 | 3,2 | 0,33 | 0,4 |
| C14:1 (n-5) | 0,9 | 0,3 | 0,2 | 0,7 | 0,78 | 0,9 |
| C15:0 | 1,2 | 1 | 0,6 | 0,6 | 0,5 | 0,6 |
| C16:0 | 21,6 | 27,6 | 17,4 | 4,2 | 0,19 | 0,2 |
| C16:1 (n-7) | 11,4 | 12,6 | 7,9 | 3,5 | 0,31 | 0,3 |
| C18:0 | 4 | 6,2 | 3,9 | 0,1 | 0,03 | 0 |
| C18:1 (n-9) | 12,5 | 15,9 | 10 | 2,5 | 0,2 | 0,2 |
| C18:1 (n-7) | 3,9 | 4,8 | 3 | 0,9 | 0,23 | 0,3 |
| C18:2 (n-6) | 1,6 | 1,6 | 1 | 0,6 | 0,38 | 0,4 |
| C18:3 (n-3) | 0,9 | 0,1 | 0,1 | 0,8 | 0,89 | 1 |
| C18:4 (n-3) | 2,7 | 1,6 | 1 | 1,7 | 0,63 | 0,7 |
| C20:0 | 0,6 | 1 | 0,6 | 0 | 0 | 0 |
| C20:1 (n-11) | 1,8 | 2,5 | 1,6 | 0,2 | 0,11 | 0,1 |
| C20:4 (n-6) | 1,1 | 0,7 | 0,4 | 0,7 | 0,64 | 0,7 |
| C20:4 (n-3) | 0,8 | 0,5 | 0,3 | 0,5 | 0,63 | 0,7 |
| C20:5 (n-3) | 15,4 | 8 | 5 | 10,4 | 0,68 | 0,8 |
| C22:5 (n-6) | 0,6 | 0,2 | 0,1 | 0,5 | 0,83 | 0,9 |
| C22:5 (n-3) | 0,9 | 0,7 | 0,4 | 0,5 | 0,56 | 0,6 |
| C22:6 (n-3) | 9,1 | 4,5 | 2,8 | 6,3 | 0,69 | 0,8 |
Table 6: Analysis of fatty acids in unreacted wax esters from sardine oil and oleyl alcohol. Lipase from Candida antarctica. Column 2 shows the FFA content before hydrolysis. Column 3 shows FFA after 18 hours. Conversion was 100–63=37%. Further columns show data elaboration (see Discussion).
Moreover, tables report the amount of unreacted wax esters after these times, as measured by HPLC/ELSD analysis of the crude reaction mixture (1: totally unreacted wax esters, 0: totally hydrolyzed wax esters). Values obtained by such analysis are fairly confirmed by weights of unreacted wax esters isolated by short column chromatography. These are usually 5 to 10% less than values obtained by HPLC, due to loss in the column.
Data obtained were subjected to kinetic analysis. The assumption is made that gas chromatographic areas represent the weight percentage of single fatty acid methyl esters (FAME) from wax esters in the mixture. Actually, it is known that this is not true, since a correction factor should be introduced by using standards of all FAME in the mixture. However, the error introduced by injecting nineteen standards for each methyl ester plus an additional internal standard could have been higher than the error introduced by such an assumption [16- 18]. Anyway, since relative rates were measured, using areas and relative differences did not change conclusions on different reactivity.
Moreover, it is assumed that the concentration of single wax esters in the system is proportional to the concentration of FAME obtained from wax ester split by hydrochloric acid/ methanol. Both assumptions are summarized by the following expression:
\[{[WE]_i} \approx {\rm{ }}GCare{a_{FAMEi}}\]
From this assumption, chromatographic areas are hereinafter referred to as concentrations. Fourth columns of tables 1-6 calculate the amount of single fatty acids in unreacted wax esters after the reported reaction times. Calculation is performed by multiplying the found FA (fatty acids) concentration in the unreacted wax ester fraction (third columns) by the residual wax ester fraction, indicated in single tables. These values are labelled in tables as “corrected areas”. Differences between values of fourth and second columns, respectively, indicate the difference in concentration of single fatty acids lost in hydrolysis and are reported in the fifth columns of tables 1-6.
When the same enzyme catalyses the same kind of reaction for several substrates, each substrate acts as an inhibitor towards all other substrates. This concept was the basis for introducing the idea of enzyme competitive kinetics [19], particularly useful in the case of lipases [20].The theory of enzyme competitive kinetics developed the specificity constant α, defined as kcat/ KM, derived from Michaelis-Menten equation, where kcat is the rate constant for irreversible decomposition of the substrateenzyme complex ES and KM is the Michaelis-Menten constant [21]. For multiple n substrates competing for reacting with the enzyme, there are n values for specificity constants α. When concentrations of competing substrates are sufficiently lower than the corresponding KM, relative rates of reaction of two substrates, A and B, are related to their concentrations and specificity constants through the following expression:
\[\frac{{{v_a}}}{{{v_b}}} = \frac{{{k_{ca{t_a}}}}}{{{k_{{M_a}}}}} * \frac{{{k_{{M_b}}}}}{{{k_{ca{t_b}}}}} * \frac{{[A]}}{{[B]}} = \frac{{{\alpha _a}}}{{{\alpha _b}}} * \frac{{[A]}}{{[B]}}\]
In the case of initial rates, the expression is
\[\frac{{{v_{{0_a}}}}}{{{v_{{0_b}}}}} = \frac{{{\alpha _a}}}{{{\alpha _b}}} * \frac{{{{[A]}_0}}}{{{{[B]}_0}}}\]
\[\frac{{{v_{{0_a}}}}}{{{{[A]}_0}}} * \frac{{{{[B]}_0}}}{{{v_{{0_b}}}}} = \frac{{{\alpha _a}}}{{{\alpha _b}}}\,\,\,(1)\]
Column 1 in tables 1-6 represents two sets of acid moieties condensed with either alcohol C22:0 or C18:1. The corresponding wax esters are a set of substrates for the three lipases used in the experiments. Each of them acts as a competing substrate towards all other ones. Moreover, they were exposed to lipases for the same time. According to the initial assumption, columns of tables 1-6 labeled as “delta area %” represent a difference of concentration for each fatty acid moiety occurred during the same time, and can be therefore considered as actual v0 . When this value is divided by the initial concentration, the value obtained and reported in columns labeled as “kapp=delta/area t0 ”, corresponds to
\[\frac{{{v_{{0_n}}}}}{{{{[N]}_0}}}\]
Finally, column 7 in tables 1-6 reports values of columns 6 divided by the highest value of the same columns. Such values, labeled as kapp rel, represent the ratio between the specificity constants of individual wax esters and the most reactive one. According to equation 1, these values represent ratios between specificity constants for each pair of substrates.
For a first qualitative appraisal of data from last columns, they were labeled with different cell colors. White cells indicate values under a certain value, whereas red cells label data from and beyond the limit value. Such limit values are different for each Table, namely 0.4, 0.6, 0.5, 0.4, 0.6, and 0.6 for tables 1-6, respectively. It is easy to see that lower kapp rel are concentrated in the upper part of tables, whereas higher ones are concentrated in the lower part. This suggests that the chain length of free fatty acid moieties plays a role in enhancing reactivity of wax esters in lipase catalyzed hydrolysis.
Data from tables 1 to 6 were analyzed by multiple linear correlation. The method used is here exemplified with data referred to porcine pancreas lipase. The same procedure was used in the case of the two other lipases, for which only final results will be given. Table 7 rearranges data of kapp from tables 2 and 5, by collecting data for both starting mixtures of wax esters and listing kapp. kapp rel were recalculated by dividing kapp’s by the highest value in the whole set, regardless of the starting wax ester. Multiple linear regression analysis was applied by checking any relationship describing kapp rel as a linear function of the following variables: number of carbon atoms in the acid moiety, number of unsaturations on the same moiety, and a dummy variable (qualitative indicator variable [22]), namely 0 and 1, labeling the fatty alcohol as C22:0 and C18:1, respectively. Obviously, it was not possible to use a variable defining the chain length of the alcohol and another one defining the number of unsaturation of the same alcohols, since these sets are perfectly multicollinear. With three variables, seven linear combinations are possible, including the three relationships between kapp rel and each of the four single variables. Out of these seven linear combinations, only one showed statistically significant results. Interestingly, this was the same relationship four the three lipases. Namely, the relationship was of the form
| k app rel | nCacid | nΔacid | Nature of fatty alcohol | |
| C14:0 | 0,22 | 14 | 0 | 0 |
| C14:1 (n-5) | 0,52 | 14 | 1 | 0 |
| C15:0 | 0,52 | 15 | 0 | 0 |
| C16:0 | 0,25 | 16 | 0 | 0 |
| C16:1 (n-7) | 0,07 | 16 | 1 | 0 |
| C18:0 | 0,27 | 18 | 0 | 0 |
| C18:1 (n-9) | 0,49 | 18 | 1 | 0 |
| C18:1 (n-7) | 0 | 18 | 1 | 0 |
| C18:2 (n-6) | 0,59 | 18 | 2 | 0 |
| C18:3 (n-3) | 0,7 | 18 | 3 | 0 |
| C18:4 (n-3) | 0,63 | 18 | 4 | 0 |
| C20:0 | 0,47 | 20 | 0 | 0 |
| C20:1 (n-11) | 0,89 | 20 | 1 | 0 |
| C20:4 (n-6) | 0,63 | 20 | 4 | 0 |
| C20:4 (n-3) | 0,7 | 20 | 4 | 0 |
| C20:5 (n-3) | 0,63 | 20 | 5 | 0 |
| C22:5 (n-6) | 1 | 22 | 5 | 0 |
| C22:5 (n-3) | 0,85 | 22 | 5 | 0 |
| C22:6 (n-6) | 0,88 | 22 | 6 | 0 |
| C14:0 | 0,48 | 14 | 0 | 1 |
| C14:1 (n-5) | 0,81 | 14 | 1 | 1 |
| C15:0 | 0,6 | 15 | 0 | 1 |
| C16:0 | 0,32 | 16 | 0 | 1 |
| C16:1 (n-7) | 0,48 | 16 | 1 | 1 |
| C18:0 | 0,16 | 18 | 0 | 1 |
| C18:1 (n-9) | 0,38 | 18 | 1 | 1 |
| C18:1 (n-7) | 0,4 | 18 | 1 | 1 |
| C18:2 (n-6) | 0,46 | 18 | 2 | 1 |
| C18:3 (n-3) | 0,58 | 18 | 3 | 1 |
| C18:4 (n-3) | 0,66 | 18 | 4 | 1 |
| C20:0 | 0 | 20 | 0 | 1 |
| C20:1 (n-11) | 0,93 | 20 | 1 | 1 |
| C20:4 (n-6) | 0,67 | 20 | 4 | 1 |
| C20:4 (n-3) | 0,66 | 20 | 4 | 1 |
| C20:5 (n-3) | 0,66 | 20 | 5 | 1 |
| C22:5 (n-6) | 0,86 | 22 | 5 | 1 |
| C22:5 (n-3) | 0,46 | 22 | 5 | 1 |
| C22:6 (n-3) | 0,68 | 22 | 6 | 1 |
Table 7: Rearranged data for multiple linear regression analysis, referred to porcine pancreas lipase. kapp values are from Tables 2 and 5. Kapp rel are calculated by dividing values of the kapp values by the highest value of the whole column, namely 0.96, regardless of the starting wax ester mixture. The other three columns report data on the length and number of unsaturation of the acid and the alcohol nature (docosanol or oleyl alcohol) as a dummy variable.
\[{k_{app{\rm{ }}rel}} = an{C_{acid}} + b{\Delta _{acid}}\]
where nCacid is the number of carbon atoms in the acid moiety and nΔacid is the number of double bonds in the same moiety.
All significance levels were set at p=0.05. The backward elimination method was used [23], by starting with a regression including the three predictors listed in table 7, namely the number of carbon atoms in the acid chains (nCacid), the number of unsaturations in the same chains (nΔacid), and the type of fatty alcohol as a dummy variable. Table 8 shows the statistical parameters (by Excel 2000 function LINEST) obtained for such first regression. The ratio between the coefficient for variable x3 and its standard error is far below the t value (Excel function T.DIST). Predictor x3 was then eliminated in the successive model.
| x3 | x2 | x1 | Intercept | adjusted R2=0,31684213 | |
| coefficient= | -0,00316 | 0,076955 | -0,00303 | 0,424078226 | |
| standard error= | 0,067808 | 0,024406 | 0,020367 | 0,339032868 | |
| R2 and sey: | 0,372233 | 0,208999 | #N/D | #N/D | |
| F and df: | 6,720083 | 34 | #N/D | #N/D | |
| ssreg and ssresid | 0,880614 | 1,485144 | #N/D | #N/D | |
| coefficient/st.error= | -0.04657 | 3,153148 | -0.14864 | 1,250846941 | |
| TINV=1,690924 | F.DIST=0,572587893 | ||||
Table 8: Excel output for the correlation of kapp rel (porcine pancreas lipase)) with nCacid (x1 ), nΔacid (x2 ), and the type of fatty alcohol as a dummy variable (x3). R2 : correlation coefficient. sey: standard error for y estimate. F: F-statistic. df: degrees of freedom. ssreg: regression sum of squares. ssresid: residual sum of squares. In addition, the table reports: a) the ratios between coefficients and intercept over the corresponding standard errors; b) the statistical t-function for data (Excel function TINV); c) the statistical F-function for data (Excel function F.DIST); d) the adjusted correlation coefficient.
Table 9 reports statistic parameters when kapp rel are correlated with nCacid and nΔacid. Regression parameters were calculating both by including the intercept and by setting it equal to zero. This was possible after eliminating the dummy variable x3 , which could have led to an estimated intercept between 0 and 1. Elimination of the intercept (Regression through the Origin, RTO) from a multiple linear regression is a debated issue. The most accepted opinion is that this may be possible only when the following conditions are met at the same time [24]:
| x3 | x1 | Intercept | adjusted R2=0,336319 | |
| coefficient= | 0,076955 | -0,00303 | 0,422499 | |
| standard error= | 0,024055 | 0,020075 | 0,33249 | |
| R2 and sey: | 0,372193 | 0,205999 | #N/D | |
| F and df: | 10,37482 | 35 | #N/D | |
| ssreg and ssresid | 0,880519 | 1,485239 | #N/D | |
| coefficient/st.error= | 3,19908 | -0,15081 | 1,270713 | |
| TINV=1,689572 | F.DIST=1,267289 | |||
| x2 | x1 | intercept | ||
| coefficient= | 0,057052 | 0,022194 | 0 | adjusted R2=0,878238 |
| standard error= | 0,018413 | 0,003033 | #N/D | |
| R2 and sey: | 0,88482 | 0,20775 | #N/D | |
| F and df: | 138,2767 | 36 | #N/D | |
| ssreg and ssresid | 11,93604 | 1,55376 | #N/D | |
| coefficient/st.error= | 3,098509 | 7,31837 | #N/D | |
| TINV=1,688298 | F.DIST=0,663776 | |||
Table 9: Excel output for the correlation of kapp rel (porcine pancreas lipase) with nCacid (x1 ) and nΔacid (x2 ), with intercept (above) and without intercept (regression through origin, below). R2 : correlation coefficient. sey: standard error for y estimate. F: F-statistic. df: degrees of freedom. ssreg: regression sum of squares. ssresid: residual sum of squares. In addition, the table reports: a) the ratios between coefficients and intercept over the corresponding standard errors; b) the statistical t-function for data (Excel function TINV); c) the statistical F-function for data (Excel function F.DIST); d) the adjusted correlation coefficient.
However, it must be pointed out that differences in the correlation coefficient R2 have no meaning between the two correlations, since such a parameter is calculated differently in either case and apparent improvement by setting intercept=0 is mainly a mathematical artifact. The same reference [24] warns against using correlation coefficient and its adjusted form in the case of RTO, whereas it suggests to use other statistical tests. Table 9 shows that ratios between coefficients and their standard errors are quite higher than t value in the case of RTO (bold characters). This indicates that the correlation has improved after eliminating both x3 and the intercept. This is not the case when correlation of the response with x1 and x2 still retains the intercept: here only the ratio x2 coefficient/standard error is over the t-value. This correlation was then rejected.
Finally, correlations were tested by eliminating either x1 or x2 , with and without including intercept (Tables 10 and 11). The case of ordinary correlation (with intercept) of x2 seems to give a satisfactory t-test for both the coefficient of the predictor and for the intercept. However, the correlation coefficient is very low. In the case of ordinary correlation only, not in the case of RTO, this value implies that only ca. 37% of variance is explained.
| x2 | Intercept | adjusted R2=0,354335 | |
| coefficient= | 0,07429 | 0,372923 | |
| standard error= | 0,016095 | 0,049124 | |
| R2 and sey: | 0,371785 | 0,203183 | |
| F and df: | 21,30525 | 36 | |
| ssreg and ssresid | 0,879554 | 1,486204 | |
| coefficient/st.error= | 4,615761 | 7,591406 | |
| T.DIST=1,688298 | F.DIST=0,663776 | ||
| x2 | intercept | ||
| coefficient= | 0,164887 | 0 | adjusted R2=0,705502 |
| standard error= | 0,017179 | #N/D | |
| R2 and sey: | 0,713462 | 0,323216 | |
| F and df: | 92,12755 | 37 | |
| ssreg and ssresid | 9,624455 | 3,865345 | |
| coefficient/st.error= | 9,59831 | #N/D | |
| T.DIST=1,687094 | F.DIST=N.A. | ||
Table 10: Excel output for the correlation of k app rel (porcine pancreas lipase) with nCacid (x1), with intercept (above) and without intercept (regression through origin, below). R2: correlation coefficient. sey: standard error for y estimate. F: F-statistic. df: degrees of freedom. ssreg: regression sum of squares. ssresid: residual sum of squares. In addition, the table reports: a) the ratios between coefficients and intercept over the corresponding standard errors; b) the statistical t-function for data (Excel function TINV); c) the statistical F-function for data (Excel function F.DIST); d) the adjusted correlation coefficient. In the case of RTO, the F test cannot be calculated for lack of degree of freedom.
| x1 | Intercept | adjusted R2=0,166082 | |
| coefficient= | 0,044158 | -0,27006 | |
| standard error= | 0,015264 | 0,282873 | |
| R2 and sey: | 0,188621 | 0,230912 | |
| F and df: | 8,368884 | 36 | |
| ssreg and ssresid | 0,446231 | 1,919527 | |
| coefficient/st.error= | 2,892902 | -0,95472 | |
| T.DIST=1,688298 | F.DIST=0,663776 | ||
| x1 | intercept | ||
| coefficient= | 0,029713 | 0 | adjusted R2=0,85005 |
| standard error= | 0,002019 | #N/D | |
| R2 and sey: | 0,854102 | 0,230635 | |
| F and df: | 216,6027 | 37 | |
| ssreg and ssresid | 11,52167 | 1,968128 | |
| coefficient/st.error= | 14,71743 | #N/D | |
| T.DIST=1,687094 | F.DIST=N.A. | ||
Table 11: Excel output for the correlation of kapp rel (porcine pancreas lipase) with nΔacid (x2), with intercept (above) and without intercept (regression through origin, below). R2: correlation coefficient. sey: standard error for y estimate. F: F-statistic. df:degrees of freedom. ssreg: regression sum of squares. ssresid: residual sum of squares. In addition, the table reports: a) the ratios between coefficients and intercept over the corresponding standard errors; b) the statistical t-function for data (Excel function TINV); c) the statistical F-function for data (Excel function F.DIST); d) the adjusted correlation coefficient. In the case of RTO, the F test cannot be calculated for lack of degree of freedom.
For a comparison between the correlation y=ax1 +bx2 (Table 11) and y=cx2 +constant (Table 13, lower part), what is making a difference is the F-value (Fisher test), much higher in the first case in comparison to the F-distribution function (both cells labeled with bold characters). The higher the F-value than that calculated by F-distribution function (Excel F.DIST), the lower is the probability that the model has been built up fortuitously. According to this criterion, the model based on the two variables nCacid(x1 ) and nΔacid(x2 ) appears to be more adequate [24].
As a last step, predictors nCacid and nΔacid were checked for possible collinearity. Linear correlation between the two sets of these values was calculated and the correlation coefficient was 0.540. A commonly used measurement for multicollinearity is the variance inflation factor (VIF), defined as
\[VIF = \frac{1}{{1 - {R^2}}}\]
A VIF higher than 10 is considered as a signal for high collinearity [25]. In the present case, VIF=2.17. So, it can be ruled out that the two predictors just reinforce the same correlation that could be obtained by only one of them.
A summary of regressions obtained with the three lipases is shown in figure 3.

Figure 3: Structure-reactivity relationships for the three lipases used in this study.
The two coefficients are positive for the three lipases. This indicates that for the three enzymes the hydrolysis rate is larger with long and unsaturated acid chains. More precisely, the longer and more unsaturated the acid chains, the higher the hydrolysis rate. It is interesting to note that the two coefficients for nCacid and nΔacid, respectively, have close numerical values in the case of Candida cylindracea and porcine pancreas lipases, whereas Candida antarctica lipase shows quite different values. This seems to suggest that the first two enzymes are more sensitive to the length of the acid chains, whereas Candida antarctica lipase is more sensitive to the number of unsaturations on the same chains.
To our knowledge, the present study is a first attempt to establish wax ester hydrolysis rates by competitive kinetics. Fat digestion in several marine fishes was investigated, by using fish intestinal fluid as a source for lipases [26]. The study showed that triglycerides are both hydrolyzed or reconstituted from glycerol and fatty acids four times faster than wax esters and pointed out some selectivity, namely with C18:2 and 18:3 slower than C20:4 and C20:5. Different conclusions were described in another paper, based on human pancreas lipase: there is no specific wax ester lipase, whereas lipases hydrolyzing triglycerides accept wax esters as substrates as well, although the hydrolysis rates of triglycerides are 10-50 times larger than those of wax esters [27]. Copepod Calanus helgolandicus contains wax esters, triglycerides, cholesteryl esters, and phospholipids. Experiments with 14C-labeled lipids showed that, after starvation, Calanus helgolandicus rapidly metabolizes both wax esters and triglycerides [28]. This result is in agreement with our results, since wax esters of Calanus helgolandicus are rich in polyunsaturated fatty acids.The correlation obtained, namely
\[{k_{app{\rm{ }}rel}} = 0.0222n{C_{acid}} + 0.0571{\rm{ }}n{\Delta _{acid}}\]
helps in understanding differences of wax esters digestibility: wax esters rich in EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are easily digested and represent a good source for these two acids. On the contrary, wax esters characterized by highly saturated acid moieties are responsible for steatorrhea and keriorrhea since they are not easily hydrolyzed and assimilated.
First of all, the study points out that porcine pancreas lipase were not so far from the behavior of the two microbial lipases utilized. This indicated that lipases from different sources show analogous catalytic properties, at least as to wax esters hydrolysis, even though protein structures are not so similar [29]. Porcine pancreas lipase shares several features with human pancreas lipase [30] and is therefore frequently used as a model for hydrolysis of lipids in humans. Data here obtained can thus contribute to answer the problem described in the introduction, namely why saturated wax esters are not digestible for man whereas wax esters rich in polyunsaturated, such those typical of roe, are easily digested. Statistical treatment of data still retains a part not completely explained by the correlation found. This is likely due partially to the number of analytical steps needed to assess relative rates. Moreover, the method of competitive kinetics could be affected by the different chemical structures of the sets of wax esters used in the study. Rates have been measured as initial rates, by supposing that all compounds were reacting in the quasilinear time zone. Some of them could have been out of this area. We think that the present study already gave a clue in order to understand the quite different behaviour of wax ester according to their structural feature. However, we plan to perform complete kinetics measurements on at least some pure esters built up from pure fatty acids and alcohols, in order to better define how their structure affect apparent KM and Vmax. Moreover, we plan to extend our studies on the fate of the alcohol moiety after digestion and on the biological effects generated by its metabolism. Recent observations showed that oxidation of fatty alcohol could not be the only way they are transformed: possible conversion to cholesteryl esters and 1-alkyl glycerols could be explained through the fatty alcoholaldehyde cycle [31]
Finally, our data show that the length and number of unsaturations on the acid moieties are important to improve hydrolysis rates. From a practical standpoint, both findings may give a suggestion for selecting proper synthetic wax esters as food additives or other dosage forms which could be completely digested or, possibly, to enrich natural wax esters not particularly rich in polyunsaturated fatty acids. As a possible speculation, recent findings describing anti-obesity properties of Calanus® oil [32] could be explained by a fast hydrolysis of the part of polyunsaturated wax esters contained in this oil, even though a role of the alcohol moiety of wax ester could be involved.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Capozzoli C, Galliani G, Ricotti F, Terraneo A (2018) Competitive Kinetics of Lipase Catalyzed Hydrolysis explain Differences in Digestibility of Wax Esters. J Biochem Analyt Stud 3(1): dx.doi.org/10.16966/2576- 5833.113
Copyright: © 2018 Capozzoli C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access